Units and Process Variables
| Description |
Equations |
| Units of force |
1 N1 lbf=1 kg⋅m/s2=32.174 lbm⋅ft/s2 |
| Weight |
W=mg |
| Gravitational acceleration |
g=9.8066 m/s2=32.174 ft/s2 |
Mass, volume and flow rate
| Description |
Equations |
| Mass flow rate |
m˙=dtdm |
| Volumetric flow rate |
V˙=dtdV |
| Molar flow rate |
n˙=dtdn |
| Density |
ρ=Vm=V˙m˙ |
| Specific volume |
v=mV=ρ1 |
| Molar volume |
Vm=nV=ρM |
| Specific gravity |
SG=ρrefρ |
| Description |
Equations |
| Mole and molecular wieght |
n=Mm |
| Mass fraction |
xA=mmA |
| Mole fraction |
yA=nnA |
Scaling factor of percent (%),
parts per million (ppm),
parts per billion (ppb) |
×100%×106 ppm×109 ppb |
| Average molecular weight |
M=∑ni∑mi=∑yiMi=(∑Mix1)−1 |
| Mass concentration |
ρA=VmA |
| Molar concentration |
cA=VnA |
| Molarity and molar |
1 M=1 mol/L |
| Description |
Equations |
| Pressure |
P=AF |
| Hydrostatic pressure |
P=P0+ρgh |
| Hydrostatic head |
P=ρgPh |
| Relationship between pressures |
Pabs=Patm+Pgauge |
| General manometer |
P1+ρ1gd1=P2+ρ2gda+ρmgh |
| Differential manometer |
P1−P2=(ρm−ρ)gh |
| Manometer for gas |
P1−P2=ρmgh=Ph |
| SCFM (standard cubic feet per minute) and ACFM (actual cubic feet per minute) |
Va˙=Vs˙PaPsTsTa (ideal gas) |
| Standard condition of gases |
natural gas - 59∘F,1 atm
other gas - 0∘C,1 atm |
| Description |
Equations |
| Conversion of temperature |
T(K)=T(∘C)+273.15T(∘R)=T(∘F)+459.67T(∘R)=1.8T(K)T(∘F)=1.8T(∘C)+32 |
| Conversion of temperature intervals |
1∘C=1.8∘F1∘R=1.8 K1∘F=1∘R1∘C=1.8 K |
| Description |
Equations |
| Balance equation |
Accumulation = Input - Output
+ Generation - Consumption |
| Fractional excess |
Fractional excess=nstoichnfed−nstoich |
| Fractional conversion |
Fractional conversion=nfednreacted |
| Fractional completion of limiting reactant |
Fractional completion=nfednreacted=nfed−νξ |
| Extent of reaction |
ξ=νini−ni0 |
| Extent of reaction in multiple reactions |
ni=ni0j∑νijξij |
Yield
theoretical = complete rxn, no side rxn |
Yield=ntheoreticalnactual×100 |
| Selectivity |
Selectivity=nundesiredndesired |
| Fractional excess of air (oxygen) |
Fractional excess air=nstoichnfed−nstoich |
| Quality of steam |
Quality of steam=mtotalmvapor |
| Description |
Equations |
| Nonreactive process |
−−No. unknown variablesNo. independent material balance speciesNo. other relations (process specifications)No. degrees of freedom |
Reactive process
Molecular species balance method
1 reaction system |
+−−No. unknown variablesNo. independent reactionNo. independent molecular speciesNo. other relationsNo. degrees of freedom |
Reactive process
Atomic species balance method
>1 reaction system |
−−−No. unknown variablesNo. independent reactive atomic speciesNo. independent nonreactive molecular speciesNo. other relationsNo. degrees of freedom |
Reactive process
Extent of reaction method
equilibrium problem |
+−−−No. unknown variablesNo. independent reactionNo. independent reactive speciesNo. independent nonreactive speciesNo. other relationsNo. degrees of freedom |
| Description |
Equations |
Estimations of density of liquid mixtures
1. Experimental data
2. Estimation formula
★ Volume addativity |
ρˉ1=i=1∑nρixiρˉ=i=1∑nxiρ1 |
| Incompressible approximation |
∂V^=0(∂P∂V^)T=0(∂T∂V^)P=0 |
| Volume expansivity |
β=V^1(∂T∂V^)P |
| Isothermal compressibility |
K=−V^1(∂P∂V^)T |
| Volume with change in T,P |
ln(V^1V^2)=β(T2−T1)−K(P2−P1) |
| Description |
Equations |
| Specific molar volume |
V^=nV |
Ideal gas equation of state
★ T>0∘C,P<1 atm |
PV=nRTPV^=RT |
| Standard conditions and actual conditions |
PsVs^PV=nTsT |
SCFM vs. ACFM
★ Ideal gas |
Va˙=Vs˙PaPsTsTa |
| Ideal gas condition |
T>0∘CP<1 atmV^ideal=PRT{>5 L/mol,80 ft3/lbmol>20 L/mol,320 ft3/lbmoldiatomicother |
| Description |
Equations |
| Partial pressure |
Pi=yiP |
| Dalton’s law |
∑Pi=P |
| Pure-component volume |
Vi=yiV |
| Amagat’s law |
∑Vi=V |
| Volume fraction of ideal gas |
yi=VVi |
| Description |
Equations |
| van der Waals equation of state |
P=V^−bRT−V^2a |
| Constant |
a=64Pc27R2Tc2 |
| Constant |
b=8PcRTc |
| Significance of 3 real roots |
V^highest=V^sat, vaporV^lowest=V^sat, liquidV^middle=no significance |
| Significance of real and imaginary roots |
V^real=V^gasV^imaginary=no significance |
| Description |
Equations |
| Virial equation of state |
RTPV^=1+V^B+V^2C+V^3D+⋯ |
| First order appox. of virial equation of state |
RTPV^=1+RTBP |
| Reduced temperature |
Tr=TcT |
| Reduced pressure |
Pr=PcP |
- Lookup Tc,Pc,ω
- Calculate Tr
- Estimate B by
- B0=0.083−Tr1.60.422
- B1=0.139−Tr4.20.172
- B=PcRTc(B0+ωB1)
- Substitute known values into first order approximation
| Description |
Equations |
| SRK equation of state |
P=V^−bRT−T0.5V^(V^+b)a |
| Constants |
a=0.4274R2Tc2.5/Pcb=0.08664RTc/Pc |
| Description |
Equations |
| SRK equation of state |
P=V^−bRT−V^(V^+b)αa |
| Constants |
a=0.4274(RTc)2/Pcb=0.08664RTc/Pcm=0.48508+1.55171ω−0.1561ω2Tr=T/Tcα=[1+m(1−Tr)]2 |
- Lookup Tc,Pc,ω
- Calculate a,b,m
- Determine the known
- If known T,V^
- Calculate Tr,α
- Solve from equation directly for P
- If known T,P
- Use equation and all knowns
- Use python to solve for V^
- If known P,V^
- Use equation, Tr,α, and all knowns
- Use python to solve for T
| Description |
Equations |
Compressibility
(Law of corresponding state) |
z=RTPV^ |
| Compressibility-factor equation of state |
PV^=zRT |
| Reduced temperature |
Tr=TcT |
| Reduced pressure |
Pr=PcP |
| Ideal reduced volume |
V^rideal=RTcPcV^ |
Kay’s rule of nonideal gas mixtures
Pseudocritical temperature |
Tc′=∑yiTci |
| Pseudocritical pressure |
Pc′=∑yiPci |
| Pseudoreduced temperature |
Tr′=Tc′T |
| Pseudoreduced pressure |
Pr′=Pc′P |
| Ideal pseudoreduced volume |
V^rideal=RTc′Pc′V^ |
- Lookup Tc,Pc
- If gas is HX2/He, adjust critical constant by Newton’s correlation
- Tca=Tc+8 K
- Pca=Pc+8 atm
- Calculate reduced value of two known variables from Tr,Pr,Vrideal
- Use compressibility chart to determine z
- Solve for unknowns from equation
| Description |
Equations |
| Clapeyron equation |
dtdP∗=TΔH^vVg^−Vl^1 |
| Clapeyron equation |
d(1/T)d(lnP∗)=−RΔH^v |
| Clausius-Clapeyron equation |
lnP∗=−RTΔH^v+B |
| Clausius-Clapeyron equation |
ln(P1P2)=−nRΔH^v(T21−T11) |
Antoine equation
(Vapor pressure of species) |
log10P∗=A−T+CB |
| Description |
Equations |
| Gibbs phase rule |
F=2+c−Π−r |
| Total vapor pressure of immiscible liquids |
P=∑Pi∗ |
Raoult’s law
★ Ideal gas and solution, non-dilute xA |
PA=yAP=xAPA∗(T) |
Henry’s law
★ Ideal gas and solution, dilute xA |
PA=yAP=xAHA(T) |
VLE of real gases
φ - fugacity coefficient
γ - activity coefficient |
yiφiP=xiγiP∗ |
Partition coefficient of ideal gas (Raoult’s law)
★ Ideal gas: φ=1,γ=1 |
Ki=xiyi=φiPγiPi∗=PPi∗ |
Partition coefficient of ideal gas (Henry’s law)
★ Ideal gas, Henry’s law assumptions |
Ki=PHi |
Saturation and humidity
| Description |
Equations |
| Relative saturation/humidity |
sr=PA∗(T)PA×100 |
| Molal saturation/humidity |
sm=P−PAPA |
| Absolute saturation/humidity |
sa=(P−PA)MAPAMA |
| Percent saturation/humidity |
sp=sm∗sm×100%=PA∗/(P−PA∗)PA/(P−PA)×100% |
Bubble and dew point
| Description |
Equations |
| Superheated vapor |
PA=yAP<PA∗(T) |
| Saturated vapor and dew point |
PA=yAP=PA∗(Tdp) |
| Degree of superheat |
T−Tdp |
| Bubble point temperature of mixture at constant P |
P=∑xiPi∗(Tbp) |
| Bubble point pressure of mixture at constant T |
Pbp=∑xiPi∗(T) |
| Dew point temperature of mixture at constant P |
∑Pi∗(Tdp)yi=1 |
| Dew point pressure of mixture at constant T |
Pdp=[∑Pi∗(T)yi]−1 |
| Description |
Equations |
| Kinetic energy |
Ek=21mv2 |
| Potential energy |
Ep=mgz |
| Internal energy |
U(T,V) |
| Total energy |
E=U+Ek+Ep |
| Work |
W=PΔV |
| Closed system balance |
ΔU+ΔEk+ΔEp=Q+W |
| ΔEk=0 |
Not accelerating |
| ΔEp=0 |
Not changing height |
| ΔU=0 |
No phase change, chemical reaction, temperature change |
| Q=0 |
Insulated system; adiabatic; temperature of system and surrounding the same |
| W=0 |
No moving parts, radiation, electric current, flow |
| Description |
Equations |
| Work |
W˙=W˙s+W˙fl |
| Enthalpy |
H=U+PV |
| Specific properties |
V^=mV,V^=nV |
| Open system balance |
ΔH˙+ΔE˙k+ΔE˙p=Q˙+W˙s |
| ΔEk=0 |
No acceleration; linear velocity of all streams the same |
| ΔEp=0 |
Stream entering and leaving at same height |
| Q˙=0 |
Insulated; adiabatic; system and surrounding temperature the same |
| W˙s=0 |
No moving parts |
| Friction loss |
F^=ΔU^−m˙Q˙ |
| Mechanical energy balance |
ρΔP+2Δv2+gΔz+F^=m˙W˙s |
Bernoulli equation
★ F^=0,W˙s=0 |
ρΔP+2Δv2+gΔz=0 |
| Description |
Equations |
| Internal energy |
ΔU=⎩⎪⎪⎪⎪⎨⎪⎪⎪⎪⎧=0≈0=0≈0(ideal gas)(real gas) P<10 bar(real gas) P>10 bar(condensed phases) |
| Enthalpy |
ΔH=⎩⎪⎪⎪⎪⎨⎪⎪⎪⎪⎧=0≈0=0≈V^ΔP(ideal gas)(real gas) P<10 bar(real gas) P>10 bar(condensed phases) |
Use (hypothetical) process paths to guide the use of equations.
| Description |
Equations |
| Heat capacity at constant volume |
CV(T)=(∂T∂U^)V |
| Heat capacity at constant pressure |
CP(T)=(∂T∂H^)P |
| Heat capacity correlation |
CP(T)=a+bT+cT2+dT3 |
| Heat capacity relation of condensed phases |
CP≈CV |
| Heat capacity relation of ideal gas |
CP=CV+R |
| Heat capacity of monoatmoic ideal gases |
CV=23R,CP=25R |
| Heat capacity of polyatomic ideal gases |
CV=25R,CP=27R |
Kopp’s rule
Heat capacity of compound (table B.10) |
CP,compound=∑νiCP,i |
Kopp’s rule
Heat capacity of mixture |
CP,mix=∑yiCP,i(T) |
| Change in internal energy at changing temperature |
ΔU^=∫T1T2CV(T) dT |
| Change in enthalpy at changing temperature |
ΔH^=∫T1T2CP(T) dT |
| Description |
Equations |
| Latent heat approximation of condensed phases |
ΔU≈ΔH |
| Latent heat approximation of ideal gas |
ΔUv≈ΔHv−RT |
| Description |
Equations |
| Heat of reaction of batch process |
ΔH=ξΔHrxn(T1,P1) |
| Heat of reaction of continuous process |
ΔH˙=ξΔH˙rxn(T1,P1) |
| Endothermic reaction |
ΔHrxn>0 |
| Exothermic reaction |
ΔHrxn<0 |
Hess’s law and heat of formation
“product minus reactant” |
ΔHrxn∘=i∑νiΔH^f,i∘ |
| Heat of formation conventions |
ΔH^f∘(elemental)=0 |
Hess’s law and heat of combustion
“reactant minus product” |
ΔHrxn∘=−i∑νiΔH^c,i∘ |
| Heat of combustion conventions |
ΔH^c∘(O2)=0ΔH^c∘(combustion product)=0
combustion product: COX2,HX2O,SOX2,NX2 |
Internal energy of reaction
(product ν>0; reactant ν<0) |
ΔUrxn=ΔHrxn−RTgas∑νi |
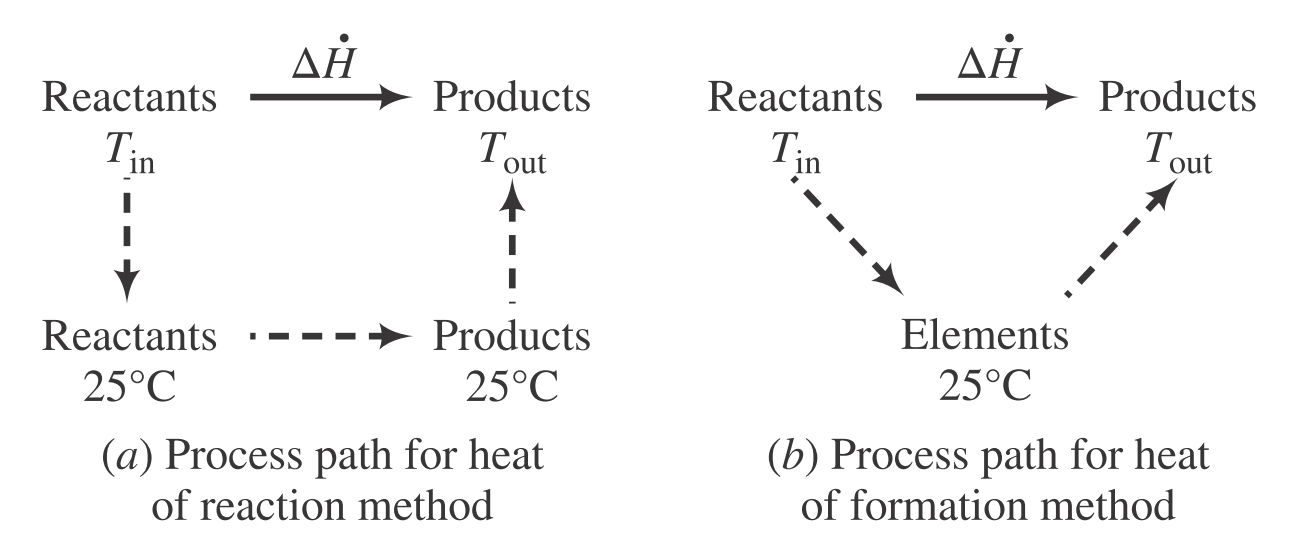
 Reaction process paths. (Elementary Principles of Chemical Processes 4e by Felder et al. p507.)
Reaction process paths. (Elementary Principles of Chemical Processes 4e by Felder et al. p507.)
| Description |
Equations |
| Enthalpy change of heat of reaction method |
ΔH˙=rxn∑ξΔHrxn∘+∑n˙outH^out−∑n˙inH^in |
| Enthalpy change of heat of formation method |
ΔH˙=∑n˙outH^out−∑n˙inH^in |