CHEM E 435 Transport Process III
Contents
Separation Processes
Types of Separations
Separations by phase creation
| Separation Operation | Feed Phase | Created Phase | Separating Agent |
|---|---|---|---|
| Partial condensation/vaporization | V and/or L | L or V | Heat transfer (ESA) |
| Flash vaporization | L | V | Pressure reduction |
| Distillation | V and/or L | V and L | Heat transfer (ESA) and sometimes shaft work (ESA) |
Separations by phase addition
| Separation Operation | Feed Phase | Created Phase | Separating Agent |
|---|---|---|---|
| Absorption | V | L | Liquid absorbent (MSA) |
| Stripping | L | V | Stripping vapor (MSA) |
| Liquid-liquid extraction | L | L | Liquid solvent (MSA) |
| Adsorption | V or L | S | Solid adsorbent (MSA) |
Separations by barrier
| Separation Operation | Feed Phase | Barrier | Separating Agent |
|---|---|---|---|
| Dialysis | L | Microporous membrane | Pressure (ESA) |
| Reverse osmosis | L | Microporous membrane | Pressure (ESA) |
| Gas permeation | Vapor | Nonporous membrane | Pressure (ESA) |
| Pervaporation | L | Nonporous membrane | Pressure and heat transfer (ESA) |
Separations by external field/gradient
- Centrifugation - pressure gradient
- Thermal diffusion - temperature gradient
- Electrolysis
- Electrodialysis - permeable membrane with fixed charge
- Electrophoresis - electric field
- Magnetophoresis - magnetic field
- Crystallization - solubility
Thermodynamics Review
| Description | Equations |
|---|---|
| Equilibrium conditions | $\begin{aligned}T_1 &= T_2 \\ P_1 &= P_2 \\ \bar{f}_2 &= \bar{f}_2 \\ a_1 &= a_2\end{aligned}$ |
| Fugacity | $\bar{f}_i = \bar{f}_i^\circ \exp\left(\dfrac{\mu_i}{RT}\right)$ |
| Fugacity coefficient | $\phi_i = \dfrac{f_i}{P}$ |
| Partial fugacity coefficient | $\phi_i = \dfrac{f_i}{x_iP}$ |
| Activity | $a_i = \dfrac{\bar{f}_i}{f_i^\circ}$ |
| Activity coefficient | $\gamma_i = \dfrac{a_i}{x_i}$ |
$K$-values
| Description | Equations |
|---|---|
| $K$-value (vapor-liquid equilibrium ratio) | $K_i = \dfrac{y_i}{x_i}$ |
| Distribution ratio (partition coefficient, liquid-liquid equilibrium ratio | $K_{D_i} = \dfrac{x_i^\alpha}{x_i^\beta}$ |
| Relative volatility | $\alpha_{i, j} = \dfrac{K_i}{K_j}$ |
| Relative selectivity | $\beta{i, j} = \dfrac{K_{D_i}}{K_{D_j}}$ |
| Raoult’s law ★ Ideal solution |
$K_i = \dfrac{P_i^*}{P}$ |
| Modified Raoult’s law ★ Low pressure |
$K_i = \gamma_{i, L}\dfrac{P_i^*}{P}$ |
| Poynting correction ★ Moderate pressure |
$K_i = \gamma_{i, L}\phi_{i, V}^\mathrm{sat}\left(\dfrac{P_i^\mathrm{sat}}{P}\right) \exp\left[\dfrac{1}{RT}\displaystyle\int^P_{P_i^{\mathrm{sat}}} v_{i, L} dP \right]$ |
| Henry’s law ★ Dilute species |
$K_i = \dfrac{H_i}{P}$ |
Mass Transfer and Diffusion
Steady-state ordinary molecular diffusion (OMD)
| Description | Equations |
|---|---|
| Fick’s first law Molecular diffusion flux of species A |
$\begin{aligned} J_{A} &= -cD_{AB}\dfrac{dx_A}{dx} \\ &= -D_{AB} \dfrac{dc_A}{dz} \end{aligned} \newline j_{A} = -\rho D_{AB}\dfrac{dw_A}{dz}$ |
| Bulk flow flux | $N = N_A + N_B$ |
| Bulk flow flux of species A | $x_A N$ |
| Molar flux of species A (definition) | $N_A = \dfrac{\dot{n}_A}{A}$ |
| Molar flux of species A ★ No eddie diffusion flux |
$\begin{aligned} N_A &= \text{bulk flow flux} + \text{molecular diffusion flux}\\ N_A &= x_A N - cD_{AB} \dfrac{dx_A}{dz} \end{aligned}$ |
| Total velocity of species A | $v_A = \dfrac{N_A}{c_A}$ |
| Molar-average mixture velocity | $v_M = v_Ax_A + v_Bx_B$ |
| Diffusion velocity of species A | $v_{D, A} = \dfrac{J_A}{c_A}$ |
| Velocity relations | $v_A = v_M + v_{D, A}$ |
Equimolar counter diffusion (EMD)
| Description | Equations |
|---|---|
| Equimolar counter diffusion | $N_A = - N_B$ |
| No bulk flow | $N = 0$ |
| Molar flux of species A Molecular diffusion flux of species A |
$N_A = J_A = -cD_{AB} \dfrac{\Delta x_A}{\Delta z}$ |
| Concentration profile of species A | $c_A = c_{A0} - \dfrac{J_A z}{D_{AB}}$ |
| Concentration profile of species B | $c_B = c_{B0} + \dfrac{J_A (z-L)}{D_{AB}}$ |
Unimolecular diffusion (UMD)
| Description | Equations |
|---|---|
| Stagnant B | $N_B = 0$ |
| Molar flux of species A Bulk flow flux |
$N = N_A$ |
| Molar flux of species A | $N_A = \dfrac{cD_{AB}}{z - z_1} \ln\left(\dfrac{1 - x_A}{1 - x_{A, 1}}\right)$ |
| Molar flux of species A | $N_A = -\dfrac{cD_{AB}}{(1 - x_A)_{\mathrm{LM}}} \dfrac{\Delta x_A}{\Delta z}$ |
| Dilute A reduces UMD to EMD (no bulk flow) | $(1 - x_A)_{\mathrm{LM}} \approx 1 \quad \text{if } x_A \to 0$ |
| Log-mean | $x_{\mathrm{LM}} = \dfrac{x_2 - x_1}{\ln(x_2/x_1)}$ |
| Composition profile | $x_A(z) = 1 - (1 - x_A) \exp\left[\dfrac{N_A (z - z_1)}{cD_{AB}}\right]$ |
| Molecular diffusion and bulk flow flux of species | $J_A = x_B N = - J_B$ |
| Changing liquid level of A with stagnant B | $N_A = -\dfrac{cD_{AB}}{(1 - x_A)_{\mathrm{LM}}} \dfrac{\Delta x_A}{z} = \dfrac{\rho_A}{\mathcal{M}_A}\dfrac{dz}{dt}$ |
Diffusivity
Diffusivity of gas mixtures
| Description | Equations |
|---|---|
| Fuller-Schettler-Giddings correlation ★ Low pressure (0 - 10 atm) ★ $D_{AB} \ [\mathrm{cm^2/s}]$ ★ $P \ [\mathrm{atm}], T \ [\mathrm{K}]$ ★ $\mathcal{M}_{AB} \ [\mathrm{g/mol}]$ |
$D_{AB} = D_{BA} = \dfrac{0.00143 T^{1.75}}{P \mathcal{M}_{AB}^{1/2} \left[(\sum_V)_A^{1/3} + (\sum_V)_B^{1/3}\right]^2} \newline $ |
| Effective molecular mass | $\mathcal{M}_{AB} = \dfrac{2}{\dfrac{1}{\mathcal{M}_A} + \dfrac{1}{\mathcal{M}_B}}$ |
| Summation of atomic and structural diffusion volumes (Table 3.1) | $\sum_V$ |
| Takahashi correlation ★ High pressure (Figure 3.3) |
$\dfrac{D_{AB} P}{(D_{AB}P)_{\text{low }P}} = \mathrm{const}$ |
| Molar averaged reduced properties | $T_r = x_A T_{r, A} + x_B T_{r, B} \newline P_r = x_A P_{r, A} + x_B P_{r, B}$ |
Diffusivity of nonelectrolyte liquid mixtures
| Description | Equations |
|---|---|
| Stoke-Einstein equation ★ Infinite dilution |
$(D_{AB})_\infty = \dfrac{RT}{6\pi \mu_B R_A N_A} \propto \dfrac{T}{\mu_B R_A}$ |
| Wilke-Chang equation ★ Low pressure (0 - 10 atm) ★ $D_{AB} \ [\mathrm{cm^2/s}]$ ★ $\mu_B \ [\mathrm{cP}], T \ [\mathrm{K}]$ ★ $\mathcal{M}_{AB} \ [\mathrm{g/mol}]$ ★ $v_A \ [\mathrm{cm^3/mol}]$ |
$(D_{AB})_\infty = 7.4\times 10^{-8} \dfrac{(\phi_B \mathcal{M}_B)^{1/2}T}{\mu_B v_A^{0.6}}$ |
| Hayduk-Minhas correlation (Modified Wilke-Chang equation) |
$(D_{AB})_\infty = 13/3\times 10^{-8} \dfrac{T^{1.47}\mu_B^\varepsilon}{v_A^{0.71}} \newline \varepsilon = \dfrac{10.2}{v_A} - 0.791$ |
Film theory for mass transfer at fluid-fluid interface
Single film theory
| Description | Equations |
|---|---|
| Concentration and mole fraction | $c_A = x_Ac$ |
| Mass transfer coefficient (no bulk flow) | $k_c = \dfrac{D_{AB}}{\delta}$ |
| Mass transfer coefficient (with bulk flow) | $k'_c = \dfrac{D_{AB}}{\delta (1 - x_A)_{\mathrm{LM}}}$ |
| Molar flux of species A ★ Interface (i), bulk (b) |
$N_A = \pm k_c (c_{A, i} - c_{A, b}) \newline N_A = \pm k'_c (c_{A, i} - c_{A, b})$ |
| Film thickness | $\delta = \dfrac{D_{AB}}{k_c} = \dfrac{D_{AB}}{k'_c (1 - x_A)_{\mathrm{LM}}}$ |
Mass transfer coefficient and driving force
| Driving Force | Mass Transfer Coefficient | Equations |
|---|---|---|
| Concentration (liquid) | $k_c \ [\mathrm{m/s}]$ | $N_A = \pm k_c (c_{A, i} - c_{A, b})$ |
| Pressure (vapor) | $k_p \ [\mathrm{mol/m^2 \cdot s \cdot Pa}]$ | $N_A = \pm k_p (P_{A, i} - P_{A, b})$ |
| Mole fraction (liquid) | $k_x \ [\mathrm{mol/m^2 \cdot s}]$ | $N_A = \pm k_x (x_{A, i} - x_{A, b})$ |
| Mole fraction (vapor) | $k_y \ [\mathrm{mol/m^2 \cdot s}]$ | $N_A = \pm k_y (y_{A, i} - y_{A, b})$ |
| Description | Equations |
|---|---|
| Liquid phase coefficient conversion | $k_x = k_c c = k_c \dfrac{\rho_L}{\mathcal{M}_L}$ |
| Vapor phase coefficient conversion | $k_y = k_p P = (k_c)_g c = (k_c)_g \dfrac{P}{RT} = (k_c)_g \dfrac{\rho_G}{\mathcal{M}_G}$ |
| Liquid phase bulk flow correction | $k' = \dfrac{k}{(1 - x_A)_{\mathrm{LM}}}$ |
| Gas phase bulk flow correction | $k' = \dfrac{k}{(1 - y_A)_{\mathrm{LM}}}$ |
Two-film theory
| Based on | Overall Mass Transfer Coefficient | Equations | Fictitious properties |
|---|---|---|---|
| Liquid phase | $\dfrac{1}{K_L} = \dfrac{H_A}{k_p} + \dfrac{1}{k_c}$ | $N_A = \pm K_L (c_A^* - c_{A, b})$ | $c_A^* = P_{A, b} H_A$ |
| Gas phase | $\dfrac{1}{K_G} = \dfrac{1}{k_p} + \dfrac{1}{H_A k_c}$ | $N_A = \pm K_G (P_{A, b} - P_A^*)$ | $P_A^* = \dfrac{c_{A, b}}{H_A}$ |
| Liquid phase mole fraction | $\dfrac{1}{K_x} = \dfrac{1}{K_a k_y} + \dfrac{1}{k_x}$ | $N_A = \pm K_y (y_{A, b} - y_{A}^*)$ | $y_A^* = x_{A, b}K_A$ |
| Gas phase mole fraction | $\dfrac{1}{K_y} = \dfrac{1}{k_y} + \dfrac{K_A}{k_x}$ | $N_A = \pm K_x (x_{A}^* - x_{A, b})$ | $x_A^* = \dfrac{y_{A, b}}{K_A}$ |
Single Equilibrium Stages
Degrees of freedom
| Description | Equations |
|---|---|
| Gibbs' phase rule | $\mathrm{DOF = component - phase + 2}$ |
| Degree of freedom | $\small\text{DOF = \# intensive variables} - \text{\# independent eqns}$ |
| Azeotrope | $x_i = y_i$ |
| $q$-line | $y_H = \left[\dfrac{(V/F) - 1}{(V/F)}\right] x_H + \left[\dfrac{1}{(V/F)}\right] z_H$ |
Rachford-Rice algorithm for isothermal flash calculation
Symbol Conventions
- Molar flow rates
- $F$ - Feed
- $V$ - Output vapor
- $L$ - Output liquid
- Molar fraction of component i
- $x_i$ - In the liquid
- $y_i$ - In the vapor
- $z_i$ - Overall
| Description | Equations |
|---|---|
| Road map | Known - $K_i, z_i, F$ Solve - $\Psi \to V \to L \to x_i, y_i \to Q$ |
| Vapor-to feed ratio | $\Psi \equiv \dfrac{V}{F}$ |
| Objective function ★ Derived from $\sum y_i - \sum x_i = 0$ |
$f(\Psi) = \displaystyle\sum_i \dfrac{z_i (1 - K_i)}{[1 + \Psi(K_i - 1)]} = 0$ |
| Derivative of objective function | $f'(\Psi) = \displaystyle\sum_i \dfrac{z_i (1 - K_i)^2}{[1 + \Psi(K_i - 1)]^2}$ |
| Newton’s method for root finding | $\Psi_{n+1} = \Psi_n - \dfrac{f(\Psi_n)}{f'(\Psi_n)}$ |
| Vapor molar flow rate | $V = \Psi F$ |
| Liquid molar flow rate | $L = F - V$ |
| Vapor mole fraction | $x_i = \dfrac{z_i}{1 + \Psi(K_i - 1)}$ |
| Liquid mole fraction | $y_i = \dfrac{z_i K_i}{1 + \Psi(K_i - 1)}$ |
| Heat | $Q = h_V V + h_L L - h_F F$ |
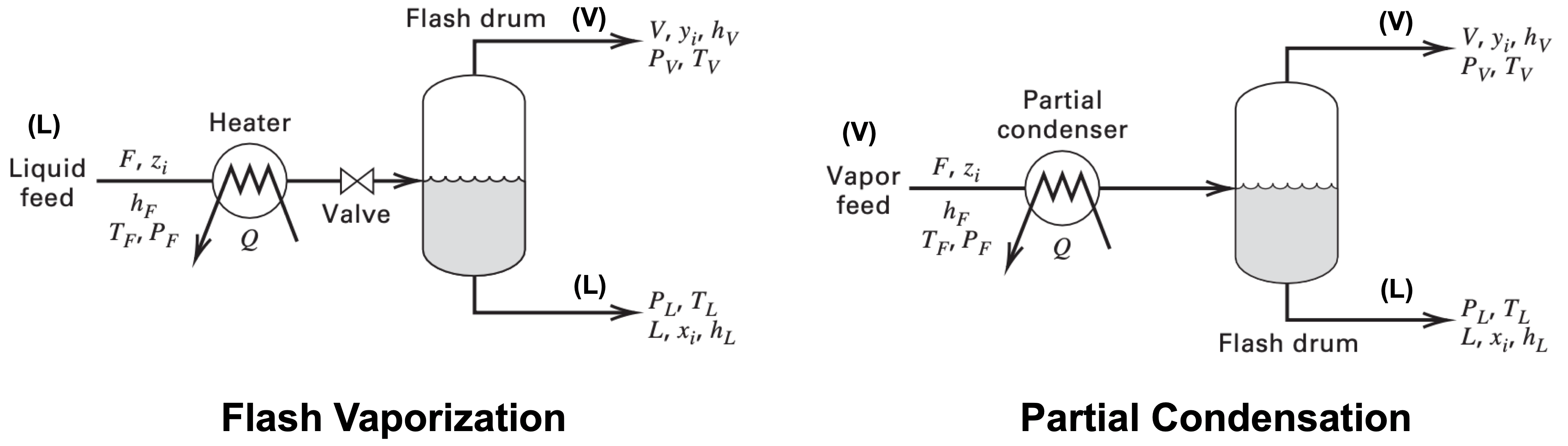
Bubble and dew point calculations
| Description | Equations |
|---|---|
| Bubble point | $\Psi = 0 \newline \sum z_i K_i = 1 \newline \sum \dfrac{y_i}{P_i^* (T)} = \dfrac{1}{P}$ |
| Dew point | $\Psi = 1 \newline \sum \dfrac{z_i}{K_i} = 1 \newline \sum x_i P_i^* (T) = P$ |
Ternary liquid-liquid extraction
Symbol Conventions
- Components
- $A$ - Carrier (feed solvent)
- $B$ - Solute
- $C$ - Solvent
- Mass flow rates
- $F$ - Feed
- $S$ - Solvent
- $E$ - Extract (Majority of solvent $C$ carrier extracted solute $B$)
- $R$ - Raffinate
- Mass fraction of component i
- $x_i$ - In the liquid
- $y_i$ - In the vapor
- $z_i$ - Overall
- Mass ratio of component i
- $X_i$ - In the liquid
- $Y_i$ - In the vapor
| Description | Equations |
|---|---|
| Mass fraction | $x_i = \dfrac{m_i}{m}$ |
| Mass ratio | $X_i = \dfrac{m_i}{m - m_i}$ |
| Conversion between mass fraction and ratio | $X_i = \dfrac{x_i}{1 - x_i} \newline x_i = \dfrac{X_i}{1 - X_i}$ |
| Solute balance ★ Solvent A, Solute B ★ Feed F, Solvent S, Extract E, Raffinate R |
$\begin{aligned} F_B &= E_B + R_B & \\ X_{F, B} F_A &= Y_{E, B} S + X_{R, B}F_A & Y_{E, B} = K'_{D_B} X_{R, B} \\ X_{F, B}F_A &= K'_{D_B} X_{R, B}S + X_{R, B}F_A & \\ X_{R, B} &= \dfrac{X_{F, B} F_A}{F_A + K'_{D_B} S} \end{aligned}$ |
| Extraction factor of solute B | $\mathcal{E} = \dfrac{K'_{D_B} S}{F_A}$ |
| Fraction of solute B unextracted | $\dfrac{X_{R, B}}{X_{F, B}} = \dfrac{1}{1 + \mathcal{E}}$ |
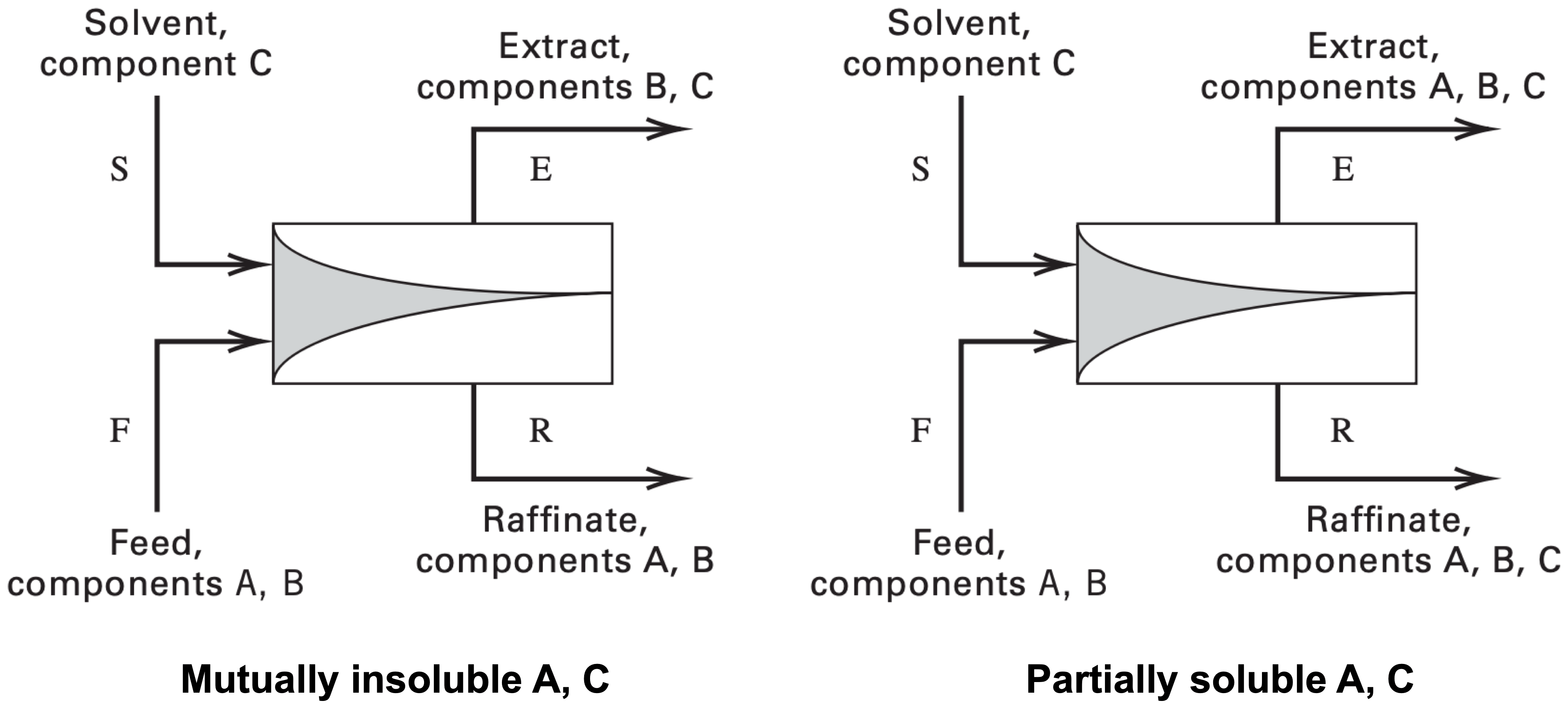
Ternary phase diagram
General principles are the same for different types of ternary phase diagrams. Here, algorithm for using equilateral ternary phase diagrams is presented.
- Identify chemical species at the apexes: feed solvent (A), feed solute (B), and pure solvent (S)
- Apexes are pure components
- Lines parallel to the apex are used to read values
- Label each side of the triangle according to the apex
- Identify compositions of entering streams: feed (F) and solvent (S)
- Assume feed only contains A and B, and solvent is pure S
- Calculate composition of mixture (M) with component balances
- $x_{M, A} = \dfrac{x_{F, A} F}{F + S}, \quad x_{M, B} = \dfrac{x_{F, B} F}{F + S}, \quad x_{M, C} = \dfrac{S}{F + S}$
- Interpolate tie line on the ternary phase diagram
- Identify compositions of exiting streams: extract (E) and raffinate (R)
- E and R lies on the intersection of tie line and miscibility boundary (binodal) curve
- E has a larger S component than R (extracted component is dissolved in solvent), being closer to point S
- Identify plait point (P)
- P lies between E and R on the miscibility boundary curve
- P the the point where E and R merge together and tie line collapses to a point
- Determine flow rate ratio with inverse level rule
- $\dfrac{E}{F + S} = \dfrac{E}{E + R} = \dfrac{\overline{MR}}{\overline{ER}}, \quad \dfrac{E}{R} = \dfrac{\overline{MR}}{\overline{ME}}$
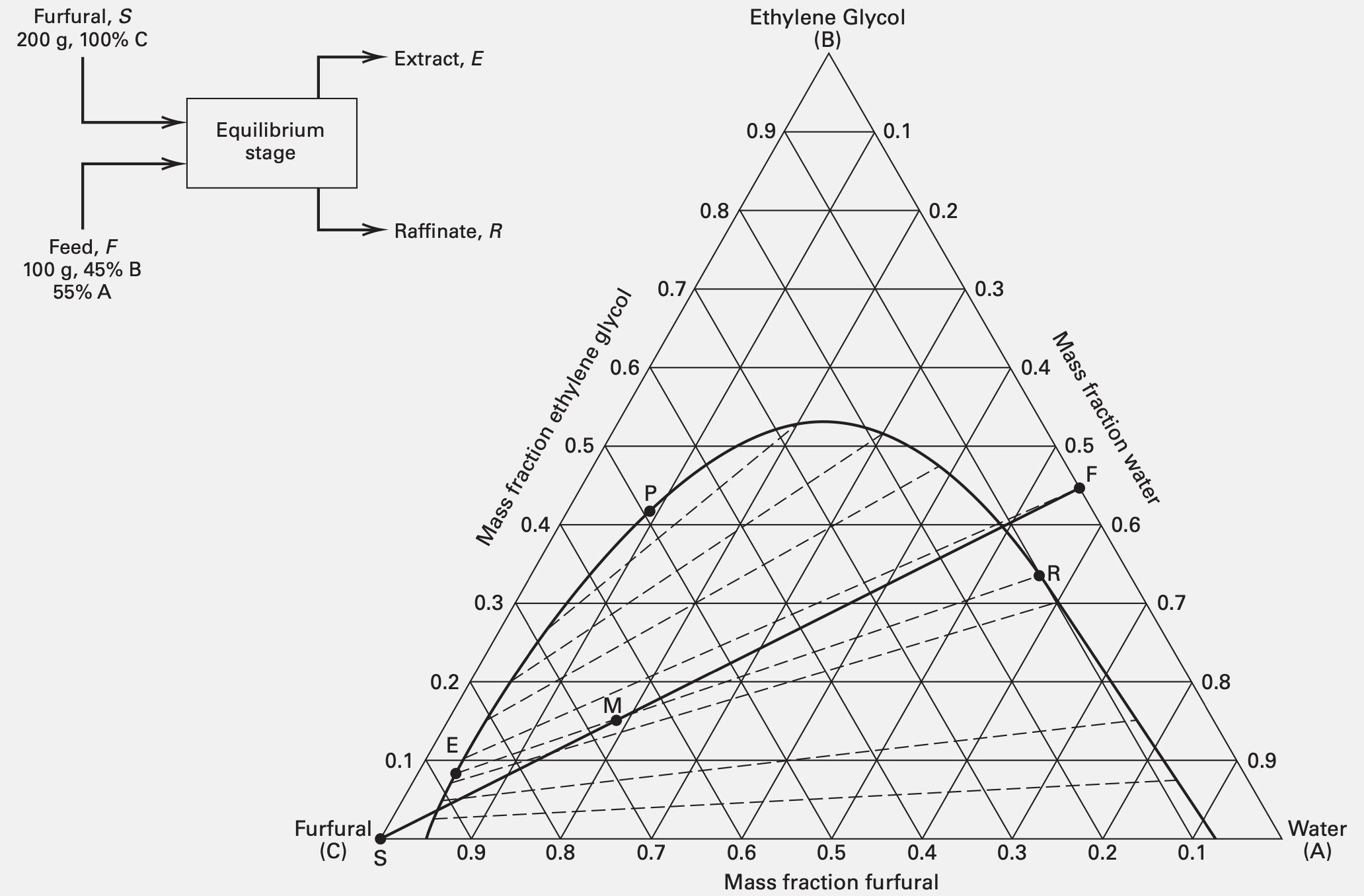
Multistage Cascades
| Configuration | Fraction of solute B unextracted |
|---|---|
| Single stage | $\dfrac{X_{R, B}}{X_{F, B}} = \dfrac{1}{1 + \mathcal{E}}$ |
| Cocurrent cascade ($N$ stages) | $\dfrac{X_{N, B}}{X_{F, B}} = \dfrac{1}{1 + \mathcal{E}}$ |
| Crosscurrent cascade ($N$ stages) | $\dfrac{X_{N, B}}{X_{F, B}} = \dfrac{1}{(1 + \mathcal{E}/N)^N}$ |
| Crosscurrent cascade ($\infty$ stages) | $\dfrac{X_{\infty, B}}{X_{F, B}} = \dfrac{1}{\exp(\mathcal{E})}$ |
| Countercurrent cascade ($N$ stages) | $\dfrac{X_{R, B}}{X_{F, B}} = \left[\displaystyle\sum_{n = 0}^N \mathcal{E}^n\right]^{-1} = \dfrac{\mathcal{E} - 1}{\mathcal{E}^{N+1} - 1}$ |
| Countercurrent cascade ($\infty$ stages) | $\begin{cases} \dfrac{X_{\infty, B}}{X_{F, B}} = 0, & \mathcal{E} \in [1, \infty) \\ \dfrac{X_{\infty, B}}{X_{F, B}} = 1 - \mathcal{E}, & \mathcal{E} \in (-\infty, 1) \end{cases}$ |
Absorption and Stripping
Graphical method for trayed towers
Symbol Conventions
- Molar flow rates
- $L$ - Total liquid feed
- $V$ - Total gas feed
- $L'$ - solute-free absorbent - constant at each stage
- $V'$ - solute-free gas (carrier gas) - constant at each stage
- Mole ratio of solute to solute-free absorbent
- $X$ - In the liquid
- $Y$ - In the vapor
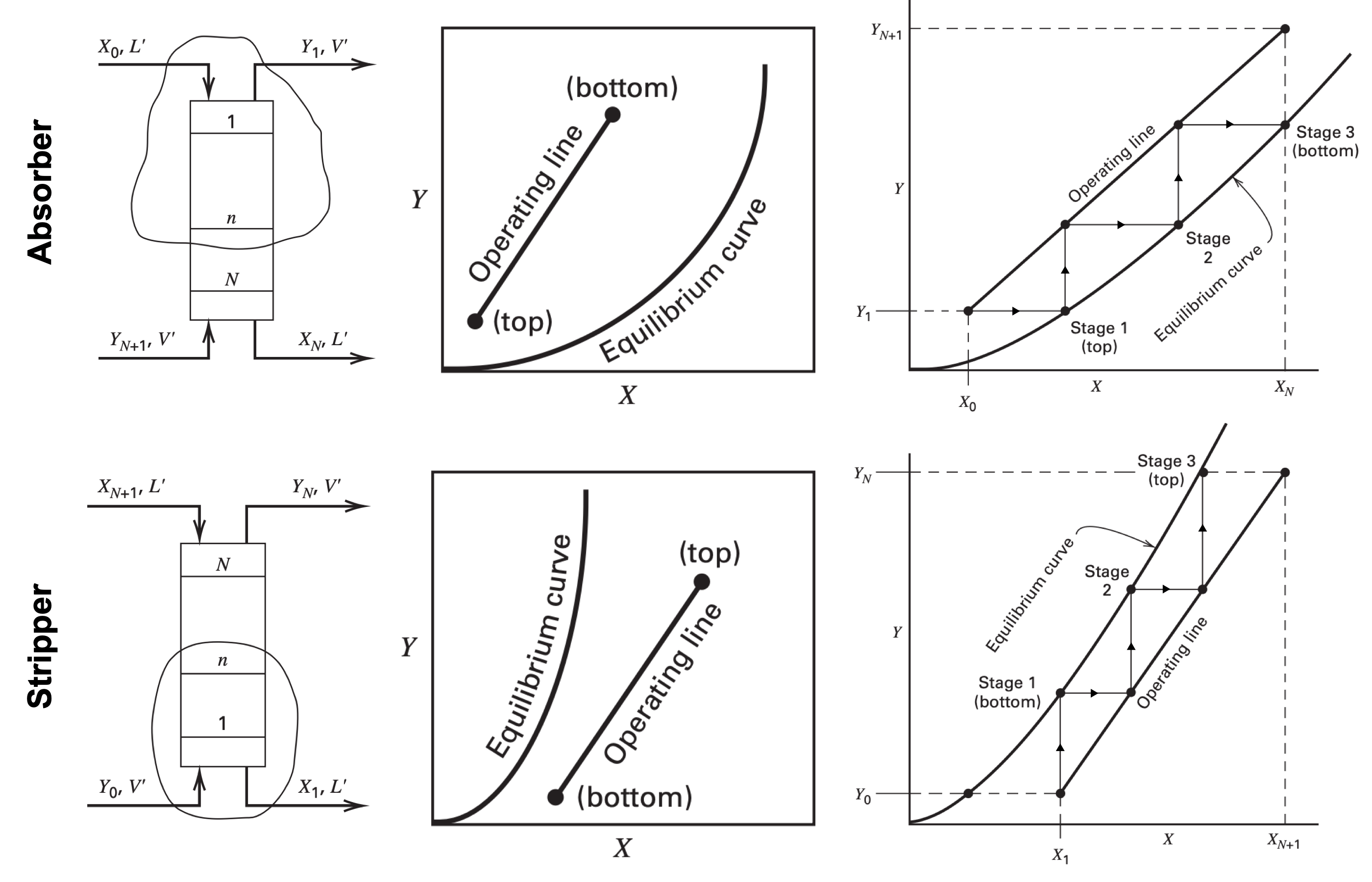
- Construct the equilibrium curve from thermodynamics
- $K_n = \dfrac{y_n}{x_n} = \dfrac{Y_n}{X_n}\dfrac{1 + X_n}{1 + Y_n} \implies Y_n = \dfrac{K_n X_n}{1 + X_n - K_n X_n}$
- Identify absorption and stripping process
- Absorption - Use liquid to remove components from gas mixture
- Stripping - Use gas to remove components from liquid mixture
- Construct operating line for $\infty$ stages for $L'_{\min}$ or $V'_{\min}$
- Absorption ($L'_{\min}$)
- Identify (liquid in, gas out) = $(X_0, Y_1)$
- Identify (liquid out, gas in) = $(X_N, Y_{N+1})$ with minimum operating line intersects and ends at the equilibrium curve
- Stripping ($V'_{\min}$)
- Identify (gas in, liquid out) = $(X_1, Y_0)$
- Identify (liquid out, gas in) = $(X_{N+1}, Y_N)$ with minimum operating line intersects and ends at the equilibrium curve
- Slope of operating line = $\dfrac{L'}{V'}$
- Absorption ($L'_{\min}$)
- Determine number of equilibrium stages for $L'$ and $V'$
- Absorption ($L'$)
- Construct operating line with point (liquid in, gas out) = $(X_0, Y_1)$ and slope = $\dfrac{L'}{V'}$
- From $(X_0, Y_1)$, draw horizontal line toward the equilibrium curve and vertical lines back to the operating line until it reaches $(X_N, Y_{N+1})$
- Stripping ($V'$)
- Construct operating line with point (gas in, liquid out) = $(X_1, Y_0)$ and slope = $\dfrac{L'}{V'}$
- From $(X_1, Y_0)$, draw horizontal line toward the equilibrium curve and vertical lines back to the operating line until it reaches $(X_{N+1}, Y_N)$
- The number of equilibrium stages is the number of intersections on the equilibrium curve
- Interpolate number of stages if not exact
- Absorption ($L'$)
Absorption
| Description | Equations |
|---|---|
| Absorption operating line | $Y_{n+1} = X_n \dfrac{L'}{V'} + Y_1 - X_0 \dfrac{L'}{V'}$ |
| Absorbent flow rate | $L' = V' \dfrac{Y_{N+1} - Y_1}{X_N - X_0}$ |
| Minimum absorbent flow rate ($\infty$ stages) | $L'_{\min} = V' K_N \cdot (\text{fraction solute absorbed})$ |
| Absorption factor | $\mathcal{A} = \dfrac{L}{K V}$ |
Stripping
| Description | Equations |
|---|---|
| Stripping operating line | $Y_{n} = X_n \dfrac{L'}{V'} + Y_0 - X_1 \dfrac{L'}{V'}$ |
| Stripping agent flow rate | $V' = L'\dfrac{X_{N} - X_1}{Y_N - Y_0}$ |
| Minimum stripping agent flow rate ($\infty$ stages) | $V'_{\min} = \dfrac{L'}{K_N} \cdot (\text{fraction solute stripped})$ |
| Stripping factor | $\mathcal{S} = \dfrac{V}{L}K$ |
| Relating stripping factor and adsorption factor | $\mathcal{S} = \dfrac{1}{\mathcal{A}}$ |
Stage efficiency and packed columns
| Description | Equations |
|---|---|
| Overall stage efficiency | $E_o = \dfrac{\text{\# theoretical stages}}{\text{\# actual stages}}$ |
| Drickamer and Bradford correlation ★ Hydrocarbons, $\mu_L \in (0.2, 1.6) \ \mathrm{cP}$ |
$E_o [\%] = 19.2 - 57.8 \log(\mu_L)$ |
| Height equivalent to a theoretical plate (HETP) | $\mathrm{HETP} = \dfrac{l_T}{N_t} = \dfrac{\text{packed height}}{\text{\# equiv eqm stages}}$ |
| Packed height | $l_T = H_{OG}N_{OG}$ |
| Overall height of a transfer unit (HTU) | $H_{OG} = \dfrac{V}{K_y a A_c}$ |
| Overall number of transfer unit (NTU) | $N_{OG} = \displaystyle\int_{y_{\text{out}}}^{y_{\text{in}}}\dfrac{dy}{y - y^*}$ |
| Overall volumetric mass transfer coefficient | $K_y a$ |
Distillation of Binary Mixtures
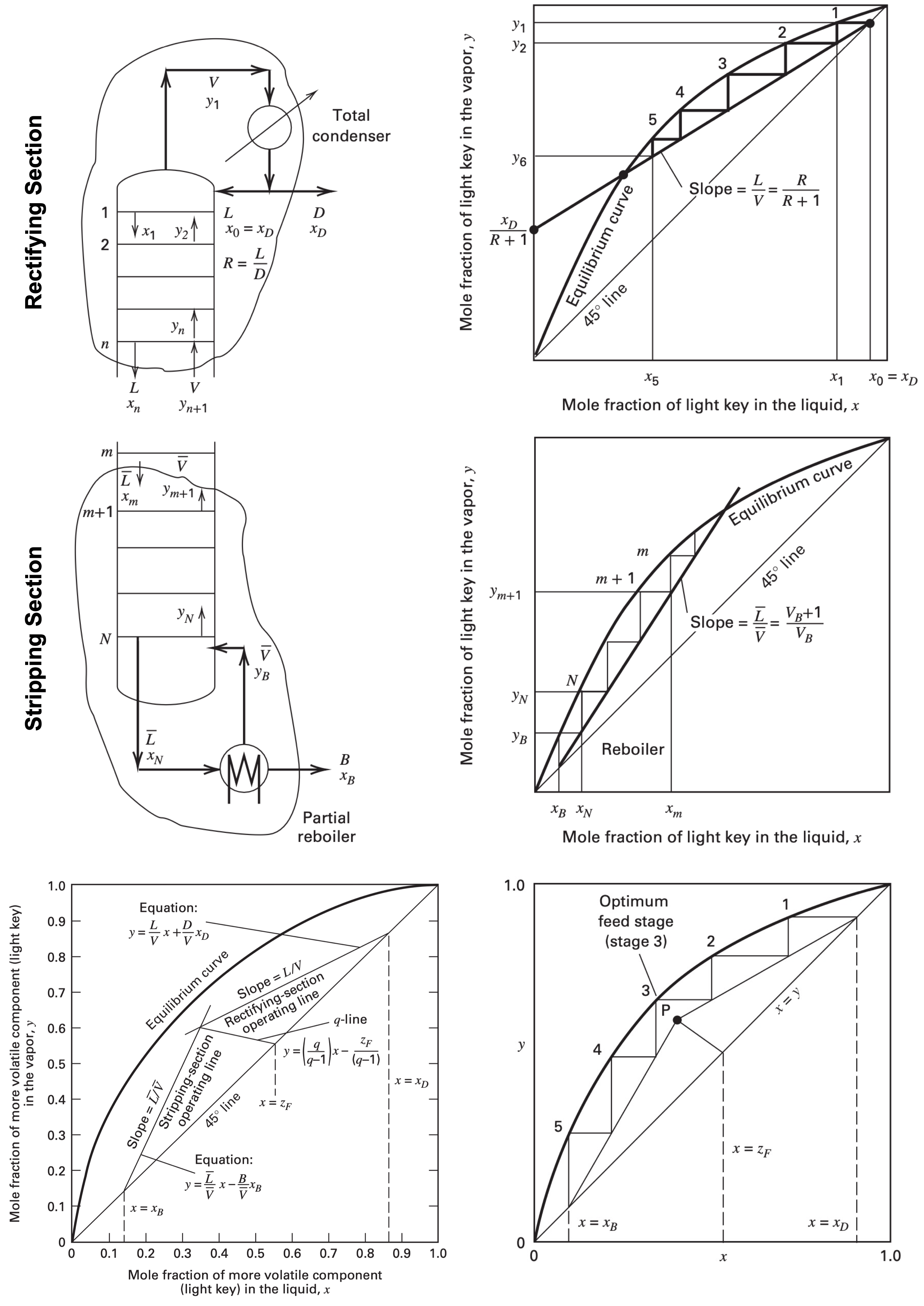
McCabe-Thiele graphical method for trayed towers
Symbol Conventions
- Molar flow rates
- $F$ - Feed
- $D$ - Distillate
- $B$ - Bottoms
- $L$ - Liquid in the rectifying section, Reflux
- $V$ - Vapor in the rectifying section
- $\overline{L}$ - Liquid in the stripping section
- $\overline{V}$ - Vapor in the stripping section, Boilup
- Mole fraction of the light key component
- $x$ - In the liquid
- $y$ - In the vapor
- $x_D$ - In the distillate
- $x_B$ - In the bottoms
- Ratio
- $\alpha_{1, 2}$ - Relative volatility
- $R$ - Reflux ratio
- $V_B$ - Boilup ratio
| Description | Equations |
|---|---|
| McCabe-Thiele assumptions | 1. Equal and constant $\Delta H_{\text{vap}}$ 2. Negligible $C_P \Delta T$ and $\Delta H_{\text{mix}}$ 3. Insulated column $q = 0$ 4. No pressure drop $\Delta P = 0$ |
| Constant molar overflow in rectifying section | $L_1 = L_i = L \newline V_1 = V_i = V$ |
| Constant molar overflow in stripping section | $\overline{L}_1 = \overline{L}_i = \overline{L} \newline \overline{V}_1 = \overline{V}_i = \overline{V}$ |
| Non-equal flow rate in rectifying and stripping sections | $L \not= \overline{L} \newline V \not= \overline{V}$ |
| Reflux ratio | $R = \dfrac{L}{D}$ |
| Boilup ratio | $V_B = \dfrac{\overline{V}}{B}$ |
| Equilibrium curve | $y_1 = \dfrac{\alpha_{1, 2}x_1}{1 + x_1(\alpha_{1, 2} - 1)}$ |
| Rectifying section operating line | $y = \left(\dfrac{R}{R + 1}\right)x + \left(\dfrac{x_D}{R + 1}\right)$ |
| Stripping section operating line | $y = \left(\dfrac{V_B + 1}{V_B}\right)x - \left(\dfrac{x_B}{V_B}\right)$ |
| $q$-parameter | $\begin{cases} q > 1 & \text{subcooled liq} \\ q = 1 & \text{saturated liq (bubble pt)} \\ q \in (0, 1) & \text{liq + vap} \\ q = 0 & \text{saturated vap (dew pt)} \\ q < 0 & \text{superheated vap} \end{cases}$ |
| $q$-parameter | $q = \dfrac{\overline{L} - L}{F} = 1 + \dfrac{\overline{V} - V}{F}$ |
| $q$-line | $y = \left(\dfrac{q}{q - 1}\right)x + \left(\dfrac{z_F}{q - 1}\right)$ |
Limiting conditions
| Description | Equations |
|---|---|
| Total reflux (Useless) |
$R_\infty = \infty \newline N_{\min} = 0 \newline L = V \newline D = B = 0$ |
| Minimum reflux | $R_{\min} = \dfrac{(L/V)_{\min}}{1 - (L/V)_{\min}} \newline N_\infty = \infty \newline V_{B, \min} = \dfrac{1}{(\overline{L}/\overline{V})_{\min} - 1}$ |
| Perfect separation | $x_D = 1, \quad x_B = 0 \newline R \ge R_{\min} \begin{cases} R_{\min} = \dfrac{1}{z_F (\alpha_{1, 2} - 1)} & q = 1 \\ R_{\min} = \dfrac{\alpha_{1, 2}}{z_F (\alpha_{1, 2} - 1)} - 1 & q = 0 \end{cases}$ |
Membrane Separations
| Description | Equations |
|---|---|
| Transmembrane molar flux | $\begin{aligned}N_i &= \dfrac{P_{M, i}}{l_M} \cdot (\text{driving force}) \\ &= \bar{P}_{M, i} \cdot (\text{driving force}) \end{aligned}$ |
| Permeance | $\bar{P}_{M, i} = \dfrac{P_{M, i}}{l_M}$ |
| Permeability | $P_{M, i} = \bar{P}_{M, i} l_M$ |
| Selectivity | $S_{i, j} = \dfrac{P_{M, i}}{P_{M, j}}$ |
| Gas permeability unit | $\begin{aligned}&1 \ \mathrm{barrer} \\ &= 10^{-10} \ \mathrm{cm^3_{STP} \cdot cm/(cm^2 \cdot s \cdot cmHg)} \\ &= 3.35 \times 10^{-16} \ \mathrm{mol \cdot m / (m^2 \cdot s \cdot Pa)} \\ &= 5.58 \times 10^{-12} \ \mathrm{lbmol \cdot ft / (ft^2 \cdot h \cdot psi)}\end{aligned}$ |
| Mole of gas in standard temperature and pressure (STP) | $1 \ \mathrm{mol} \sim 22.4 \ \mathrm{L_{STP}}$ |
Transport through porous membranes
Bulk flow
| Description | Equations |
|---|---|
| Hagen-Poiseuille flow ★ Laminar flow $\mathrm{Re} < 2100$ |
$v = \dfrac{D^2}{32\mu L}(P_0 - P_L)$ |
| Pore number density | $n = \dfrac{n_\text{total}}{A_c} = \dfrac{\text{total \# of pores}}{\text{membrane cross sectional area}}$ |
| Porosity (void fraction) ★ Straight cylindrical pore |
$\varepsilon = \frac{1}{4}n\pi D^2$ |
| Bulk flow flux ★ Straight cylindrical pore |
$\begin{aligned}N &= v\rho\varepsilon \\ &= \dfrac{\varepsilon\rho D^2}{32 \mu l_M} (P_0 - P_L) \\ &= \dfrac{n\pi\rho D^4}{128 v l_M}(P_0 - P_L)\end{aligned}$ |
| Empirical hydraulic diameter | $d_H = \dfrac{4\varepsilon}{a_v}$ |
| Specific surface area | $a_v = \dfrac{a}{1 - \varepsilon}$ |
| Total pore surface area | $a = a_v (1 - \varepsilon)$ |
| Tortuosity correction of membrane width | $\tau l_M$ |
| Bulk flow flux ★ General pore |
$N = \dfrac{\rho\varepsilon^2}{2(1-\varepsilon)^2 \tau a_v^2 \mu l_M}(P_0 - P_L)$ |
| Ergun equation | $\dfrac{P_0 - P_L}{l_M} = \dfrac{150 \mu v_0 (1-\varepsilon)^2}{D_P^2 \varepsilon^3} + \dfrac{1.75 \rho v_0^2 (1 - \varepsilon)}{D_P \varepsilon^3}$ |
| Mean spherical particle diameter | $D_P = \dfrac{6}{a_v}$ |
Liquid diffusion
| Description | Equations |
|---|---|
| Concentration driving force | $\Delta c \not= 0 \newline \Delta P = 0$ |
| Transmembrane mass flux | $N_i = \dfrac{D_{e, i}}{l_M}(c_{i, 0} - c_{i, L})$ |
| Effective diffusivity | $D_{e, i} = \dfrac{\varepsilon D_i}{\tau} K_{r, i}$ |
| Restrictive factor of solute | $K_r = \left[ 1 - \dfrac{d_m}{d_p}\right]^4$ |
| Selectivity | $S_{i, j} = \dfrac{D_i K_{r, i}}{D_j K_{r, j}}$ |
Gas diffusion
| Description | Equations |
|---|---|
| Transmembrane molar flux ★ Ideal gas, uniform transmembrane T, P |
$\begin{aligned} N_i &= \dfrac{c_M}{P}\dfrac{D_{e, i}}{l_M}(P_{i, 0} - P_{i, L}) \\ &= \dfrac{1}{RT}\dfrac{D_{e, i}}{l_M}(P_{i, 0} - P_{i, L}) \end{aligned}$ |
| Effective diffusivity | $D_{e, i} = \dfrac{\varepsilon}{\tau} \left[\dfrac{1}{(1/D_i) + (1/D_{K, i})}\right]$ |
| Knudsen diffusivity of straight cylindrical pore ★ Kinetic theory of gas |
$D_{K, i} \ [\mathrm{cm/s}] = 4850 d_p \ [\mathrm{cm}] \sqrt{\dfrac{T}{\mathcal{M}_i}\dfrac{[\mathrm{K}]}{[\mathrm{g/mol}]}}$ |
| Selectivity of Knudsen diffusion ★ $D_i \gg D_{K, i}$ ★ $D_{e, i} \sim D_{K, i}$ |
$S_{i, j} = \dfrac{P_{M, i}}{P_{M, j}} = \sqrt{\dfrac{\mathcal{M}_j}{\mathcal{M}_i}}$ |
Transport through nonporous (dense) membranes
Solution-diffusion of liquid mixtures
| Description | Equations |
|---|---|
| Thermodynamic equilibrium partition coefficient | $K_i = \dfrac{c_i}{c_i'}$ |
| Transmembrance mass flux ★ Fick’s law |
$N_i = \dfrac{D_i}{l_M}(c_{i, 0} - c_{i, L})$ |
| Transmembrance mass flux ★ $K_{i, 0} = K_{i, L} = K_{i}$ |
$N_i = \dfrac{K_i D_i}{l_M}(c'_{i, 0} - c'_{i, L})$ |
| Transmembrance mass flux ★ No mass transfer resistance in fluid boundary layers ★ $c'_{i, 0} = c_{i, F}$ and $c'_{i, L} = c_{i, P}$ |
$N_i = \dfrac{K_i D_i}{l_M}(c_{i, F} - c_{i, P})$ |
Solution-diffusion of gas mixtures
| Description | Equations |
|---|---|
| Henry’s constant | $H_i = \dfrac{c_i}{P_i}$ |
| Transmembrance mass flux ★ Fick’s law |
$N_i = \dfrac{D_i}{l_M}(c_{i, 0} - c_{i, L})$ |
| Transmembrance mass flux ★ $H_{i, 0} = H_{i, L} = H_{i}$ |
$N_i = \dfrac{H_i D_i}{l_M}(P_{i, 0} - P_{i, L})$ |
| Transmembrance mass flux ★ No mass transfer resistance in external boundary layers ★ $P_{i, 0} = P_{i, F}$ and $P_{i, L} = P_{i, P}$ |
$N_i = \dfrac{H_i D_i}{l_M}(P_{i, F} - P_{i, P})$ |
Separation factor
| Description | Equations |
|---|---|
| Ideal separation factor ★ Downstream permeate pressure negligible compared to upstream feed pressure ★ $x_{i, P} P_P \ll x_{i, F} P_F$ and $x_{j, P} P_P \ll x_{j, F} P_F$ |
$\alpha^*_{i, j} = \dfrac{H_i D_i}{H_j D_j} = \dfrac{P_{M, i}}{P_{M, j}}$ |
| Separation factor | $\alpha_{i, j} = \alpha^*_{i, j} \left[\dfrac{(x_{j, F} / x_{j, P}) - r\alpha_{i, j}}{(x_{j, F} / x_{j, P}) - r}\right]$ |
| Cut (fraction of feed permeated) | $\theta = \dfrac{\dot{n}_P}{\dot{n}_F}$ |
Summary of transport through membranes
| Type of transport | Permselective | Membrane Type | Driving Force | Permeability |
|---|---|---|---|---|
| (L) Bulk flow | No | Macroporous | $\Delta P$ | $\begin{aligned}P_M = \dfrac{\rho\varepsilon^3}{2(1 - \varepsilon)^2 \tau a_v^2 \mu}\end{aligned}$ |
| (L) Molecular diffusion | Yes | Microporous | $\Delta c$ | $P_M = D_{e, i} = \dfrac{\varepsilon D_i K_{r, i}}{\tau}$ |
| (G) Molecular diffusion ★ high P ★ $d_p > d_m$ |
Yes | Microporous | $\Delta P_i \newline \Delta P = 0$ | $P_{M, i} = \dfrac{D_{e, i}}{RT}$ |
| (G) Knudsen diffusion ★ low P ★ $d_p \sim d_m$ |
Yes | Microporous | $\Delta P_i \newline \Delta P = 0$ | $P_{M, i} = \dfrac{D_{e, i}}{RT}$ |
| (L) Solution diffusion | Yes | Nonporous (dense) | $\Delta c$ | $P_{M, i} = K_i D_i$ |
| (G) Solution diffusion | Yes | Nonporous (dense) | $\Delta P_i \newline \Delta P = 0$ | $P_{M, i} = H_i D_i$ |
Reverse osmosis
| Description | Equations |
|---|---|
| Osmotic pressure ★ Equilibrium |
$\Pi \equiv P_1 - P_{\ce{H2O}}$ |
| Osmotic pressure of water | $\Pi_{\ce{H2O}} = 0$ |
| Osmotic process | $P_1 - P_{\ce{H2O}} < \Pi$ |
| Reverse osmotic process | $P_1 - P_{\ce{H2O}} > \Pi$ |
| Osmotic pressure’s thermodynamic derivation ★ Between pure water and solution ★ Solvent $A$, solute $B$ |
$\Pi = -\dfrac{RT}{v_{A}} \ln(x_A \gamma_A)$ |
| Osmotic pressure ★ Ideal solution, dilute $B$ |
$\Pi = \dfrac{RT n_B}{v_A} = \dfrac{RTc_B}{\mathcal{M}_B}$ |
Seawater reverse osmosis
| Description | Equations |
|---|---|
| Seawater as solution 1; salt as solute B | $\ce{A} = \text{water} = \ce{H2O} \newline \ce{B} = \text{salt} = \ce{S}$ |
| Molar mass | $\mathcal{M} = \dfrac{m}{n}$ |
| Molarity (molar concentration) | $M = \dfrac{n}{V} = \dfrac{\mathrm{wt \% \ S}}{100 \% - \mathrm{wt \% \ S}}\dfrac{\rho_{\ce{H2O}}}{\mathcal{M}_S}$ |
| Seawater osmotic pressure correlation | $\Pi \ [\mathrm{psia}] = 1.12 T \ [\mathrm{K}] \sum M_i \ [\mathrm{mol/L}]$ |
| Transmembrane molar flux of water (solvent A) | $N_{\ce{H2O}} = \dfrac{P_{M, \ce{H2O}}}{l_M} (\Delta P - \Delta \pi)$ |
| Salt passage | $\mathrm{SP} = \dfrac{c_{S, P}}{c_{S, F}}$ |
| Salt rejection | $\mathrm{SR} = 1 - \mathrm{SP}$ |
| Concentration polarization factor | $\Gamma \equiv \dfrac{c_{S, i} - c_{S, F}}{c_{S, F}} = \dfrac{N_{\ce{H2O}} (\mathrm{SR})}{k_S}$ |
| Significant concentration polarization | $\Gamma > 0.2$ |