CHEM E 326 Chemical Engineering Thermodynamics
Contents
Thermodynamic Properties and Data
| Description | Equations |
|---|---|
| Mechanical equilibrium | $P_{\text{sys}} = P_{\text{surr}}$ |
| Thermal equilibrium | $T_{\text{sys}} = T_{\text{surr}}$ |
| Chemical equilibrium | $\mu(t_1) = \mu(t_2)$ |
| Gibbs phase rule | $\mathcal{F} = 2 + c - p - r$ |
| Quality | $x = \dfrac{n_v}{n_l + n_v} = \dfrac{v - v_l}{v_v - v_l}$ |
| Critical point | $\left( \dfrac{\partial P}{\partial v} \right)_{T_c} = 0, \left( \dfrac{\partial^2 P}{\partial v^2} \right)_{T_c} = 0$ |
First Law of Thermodynamics
| System Type | Equations |
|---|---|
| Closed systems | $\Delta u + \Delta e_K + \Delta e_P = q + w$ |
| Closed systems | $\Delta u = q + w$ |
| Open systems | $\dfrac{dU}{dt} = \sum\limits_{\text{in}}\dot{n}_i h_i + \sum\limits_{\text{out}}\dot{n}_i h_i + \dot{Q} + \dot{W}_s$ |
| Open system at steady state | $0 = \sum\limits_{\text{in}}\dot{n}_i h_i + \sum\limits_{\text{out}}\dot{n}_i h_i + \dot{Q} + \dot{W}_s$ |
| Description | Equations |
|---|---|
| Work | $w = - \int P_{\text{ext}} dv$ |
| Enthalpy | $h = u + Pv$ |
| Efficiency of irreversible isothermal expansion | $\eta = \dfrac{w_{\text{irrev}}}{w_{\text{rev}}}$ |
| Efficiency of irreversible isothermal compression | $\eta = \dfrac{w_{\text{rev}}}{w_{\text{irrev}}}$ |
Heat capacity
| Description | Equations |
|---|---|
| Constant volume heat capacity | $c_v = \left(\dfrac{\partial u}{\partial T}\right)_v$ |
| Constant pressure heat capacity | $c_P = \left(\dfrac{\partial h}{\partial T}\right)_P$ |
| Ideal gas heat capacity | $c_P = c_v + R$ |
| Condensed phase heat capacity (l, s) | $c_P \approx c_v$ |
| Mean heat capacity of gas | $\bar{c}_P = \dfrac{1}{T_2 - T_1} \displaystyle\int_{T_1}^{T_2} c_P(T) dT$ |
Enthalpy
| Description | Equations |
|---|---|
| Enthalpy of vaporization | $\Delta h_{\text{vap}} = h_v - h_l$ |
| Enthalpy of fusion | $\Delta h_{\text{fus}} = h_s - h_l$ |
| Enthalpy of sublimation | $\Delta h_{\text{sub}} = h_v - h_s$ |
| Enthalpy of phase change at any $T$ | $\Delta h_{\text{vap}}(T) = \Delta h_{\text{vap}}(T_b) + \int_{T_b}^{T} (c_P^{v} - c_P^l)dT$ |
| Enthalpy of reaction | $\Delta h_{\text{rxn}}^\circ = \sum \nu_i \Delta h_{f, i}$ |
Second Law of Thermodynamics
| System Type | Equations |
|---|---|
| Isolated system | $\Delta S_{\text{univ}} \ge 0$ |
| Closed system | $\Delta S_{\text{sys}} - \dfrac{Q_{\text{sys}}}{T_{\text{surr}}} \ge 0$ |
| Open system | $\sum\limits_{\text{out}} \dot{n}_i s_i - \sum\limits_{\text{in}} \dot{n}_i s_i - \dfrac{\dot{Q}}{T_{\text{surr}}} + \dfrac{dS}{dt} \ge 0$ |
| Open system at steady state | $\sum\limits_{\text{out}} \dot{n}_i s_i - \sum\limits_{\text{in}} \dot{n}_i s_i - \dfrac{\dot{Q}}{T_{\text{surr}}} \ge 0$ |
| Description | Equations |
|---|---|
| Entropy | $ds = \dfrac{\delta q_{\text{rev}}}{T}$ |
Closed System Balance
Polytropic processes
| Description | $\gamma$ | Equation |
|---|---|---|
| Polytropic | - | $PV^\gamma = \text{const}$ |
| Isobaric | $0$ | $P = \text{const}$ |
| Isothermal | $1$ | $PV = \text{const}$ |
| Isentropic | $k = \dfrac{c_P}{c_v}$ | $PV^k = \text{const}$ |
| Isochoric | $\infty$ | $V = \text{const}$ |
Isothermal/Isoenergetic process
Isoenergetic process ($\Delta u = 0 \implies \Delta T = 0$) of ideal gas has similar analysis.
| Description | Equations |
|---|---|
| Condition ★ Ideal gas |
$\Delta T = 0$ |
| Internal energy change | $\Delta u = 0$ |
| Enthalpy change | $\Delta h = 0$ |
| First law | $\Delta u = q + w = 0$ |
| Work (changing volume) | $w = -\displaystyle\int \dfrac{RT}{v} dv = -RT\ln\left(\dfrac{v_2}{v_1}\right)$ |
| Work (changing pressure) | $w = \displaystyle\int \dfrac{RT}{P} dP = RT\ln\left(\dfrac{P_2}{P_1}\right)$ |
| Heat | $q = -w$ |
| Entropy change | $\Delta s = \displaystyle\int \dfrac{\delta q}{T} = \dfrac{q}{T} = -\dfrac{w}{T}$ |
| Entropy change (changing volume) | $\Delta s = R\ln\left(\dfrac{v_2}{v_1}\right)$ |
| Entropy change (changing concentration) | $\Delta s = -R\ln\left(\dfrac{c_2}{c_1}\right)$ |
| Entropy change (changing pressure) | $\Delta s = -R\ln\left(\dfrac{P_2}{P_1}\right)$ |
Adiabatic/Isentropic process
| Description | Equations |
|---|---|
| Condition ★ Ideal gas |
$q = 0$ |
| First law | $\Delta u = w$ |
| Enthalpy change | $\Delta h = \Delta u + R \Delta T$ |
| Work (changing volume) | $w = -\displaystyle\int \dfrac{RT}{v} dv = -RT\ln\left(\dfrac{v_2}{v_1}\right)$ |
| Work (changing pressure) | $w = \displaystyle\int \dfrac{RT}{P} dP = RT\ln\left(\dfrac{P_2}{P_1}\right)$ |
| Entropy change | $\Delta s = 0$ |
| Heat capacity ratio | $\gamma = \dfrac{c_P}{c_v}$ |
| PVT relationship | $P_1 V_1^\gamma = P_2 V_2^\gamma \newline T_1 V_1^{\gamma-1} = T_2 V_2^{\gamma-1} \newline P_1^{(1/\gamma)-1} T_1 = P_2^{(1/\gamma)-1} T_2$ |
Isochoric process
| Description | Equations |
|---|---|
| Condition ★ Ideal gas |
$\Delta v = 0$ |
| Work | $w = 0$ |
| Internal energy change | $\Delta u = \displaystyle\int c_v \ dT$ |
| First law | $q = \Delta u$ |
| Entropy change | $\Delta s = \displaystyle\int \dfrac{\delta q}{T} = \int \dfrac{du}{T} = \int \dfrac{c_v}{T} \ dT$ |
Isobaric process
| Description | Equations |
|---|---|
| Condition ★ Ideal gas |
$\Delta P = 0$ |
| Internal energy change | $\Delta u = \displaystyle\int c_v \ dT$ |
| Enthalpy change | $\Delta h = \displaystyle\int c_p \ dT$ |
| Work | $w = -P\Delta v$ |
| Heat | $q = \Delta h$ |
| Entropy change | $\Delta s = \displaystyle\int \dfrac{\delta q}{T} = \int \dfrac{dh}{T} = \int \dfrac{c_p}{T} \ dT$ |
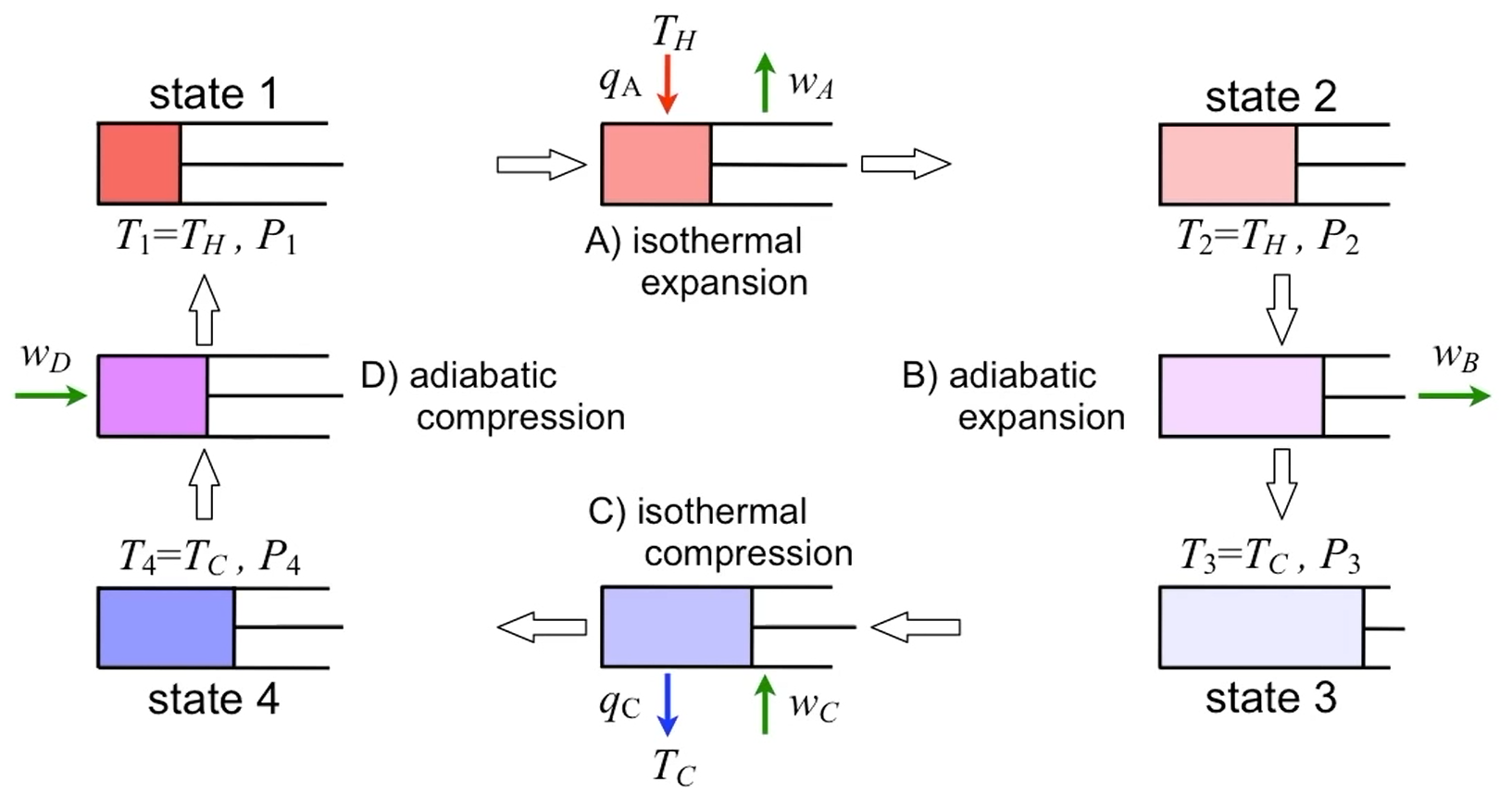
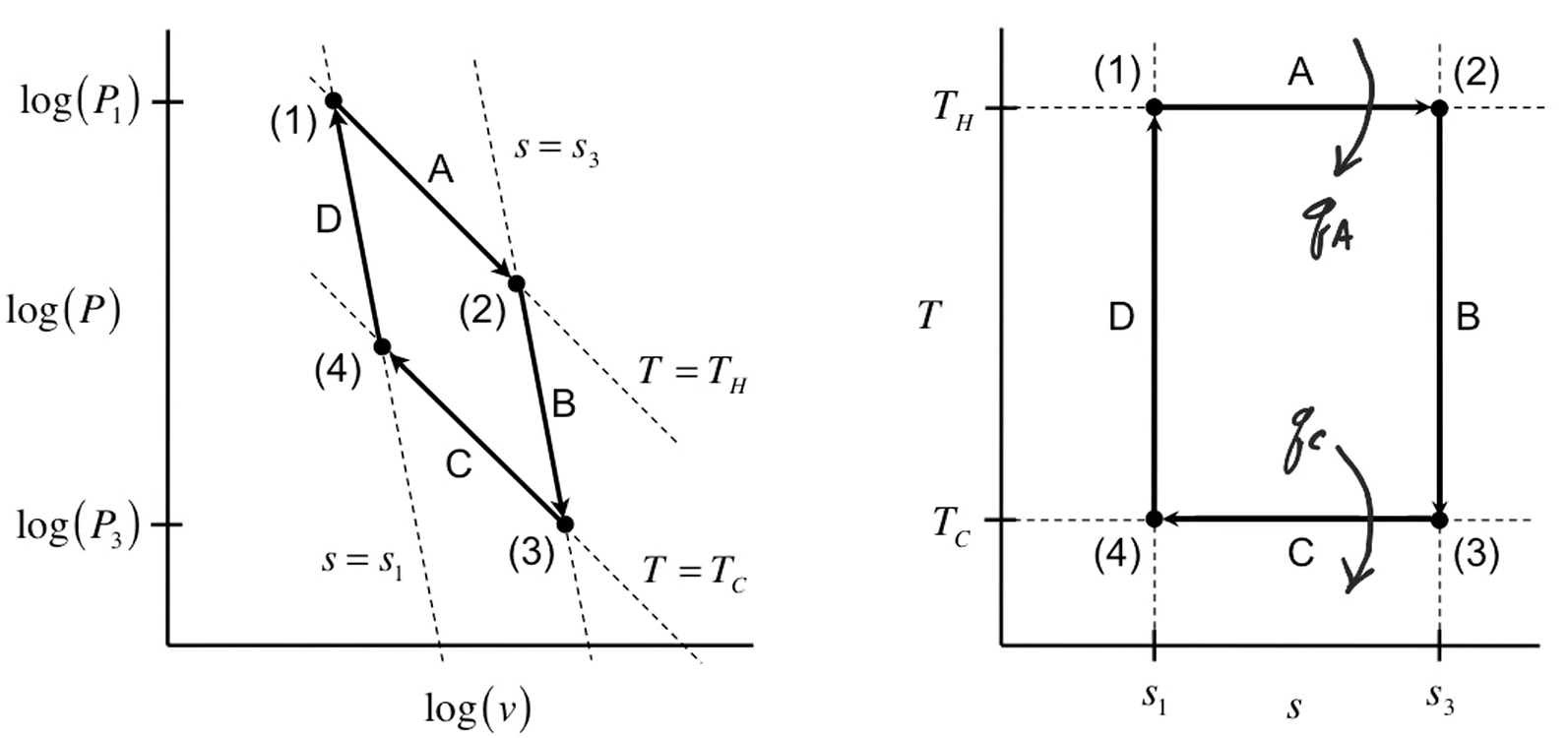
Carnot cycle
| Description | Equations |
|---|---|
| Net work | $-W_{\text{net}} = \vert W_{12} \vert + \vert W_{23} \vert - \vert W_{34} \vert - \vert W_{41} \vert$ |
| Net work and heat | $-W_{\text{net}} = \vert Q_H \vert - \vert Q_C \vert$ |
| Carnot efficiency | $\eta = 1 - \dfrac{T_H}{T_C}$ |
| State properties after cycle | $\Delta u_{\text{cycle}} = 0 \newline \Delta h_{\text{cycle}} = 0 \newline \Delta s_{\text{cycle}} = 0$ |
| Entropy change of surrounding | $\Delta s_{\text{surr}} = 0 = -\dfrac{q_H}{T_H} -\dfrac{q_C}{T_C}$ |
Open System Balance
| Description | Equations |
|---|---|
| Open system balance | $0 = \dot{m}_1 (\hat{h} + \frac{1}{2}v^2 + gz)_1 - \dot{m}_2 (\hat{h} + \frac{1}{2}v^2 + gz)_2$ |
| Shaft work | $\dot{w}_s = \int v \ dP + \Delta \dot{e}_K + \Delta \dot{e}_P$ |
| Nozzle, diffuser simplifications | $0 = \Delta E_P = \dot{Q} = \dot{W}_s$ |
| Turbine, pump, compressor simplifications | $0 = \Delta E_K = \dot{Q}$ |
| Heat exchanger simplifications | $0 = \Delta E_P = \Delta E_K = \dot{W}_s$ |
| Throttling device simplifications | $0 = \Delta E_P = \Delta E_K = \dot{Q} = \dot{W}_s$ |
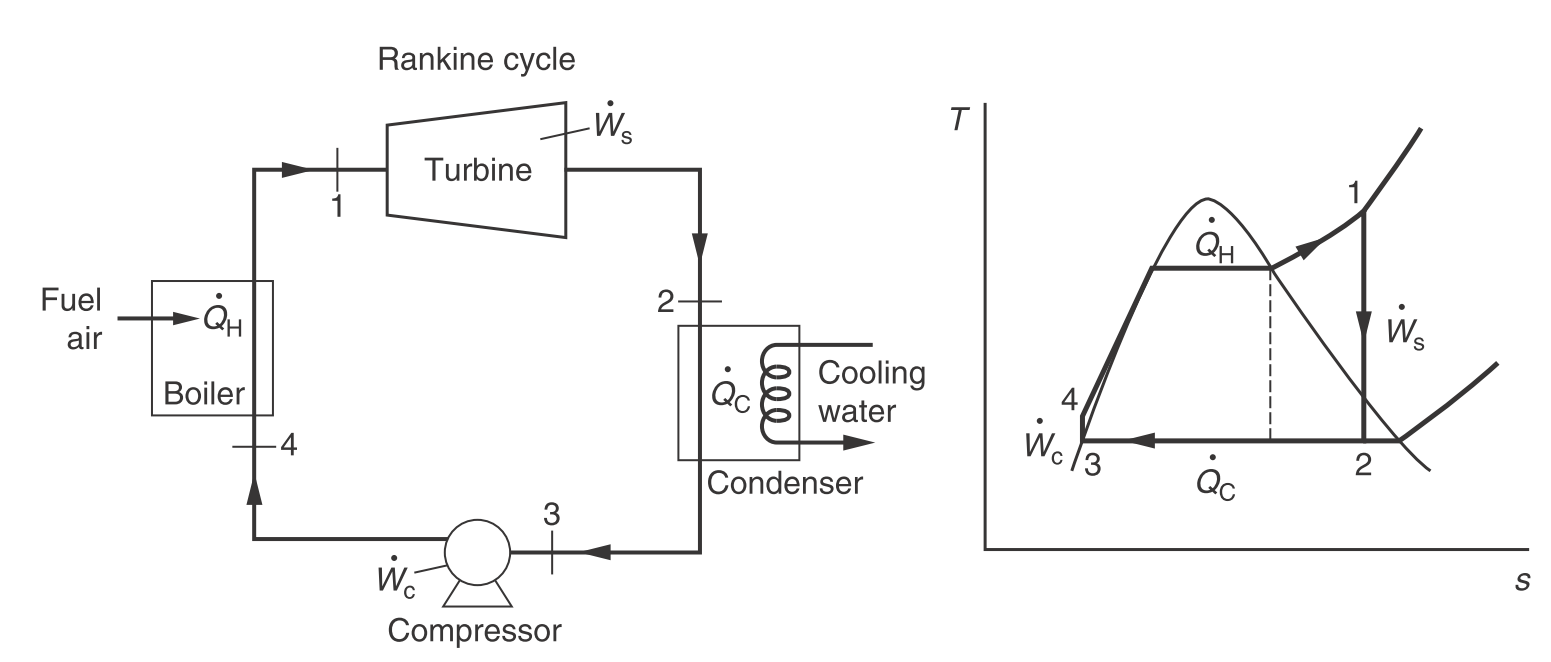
Rankine cycle
| Description | Equations |
|---|---|
| Turbine | $\dot{w}_s = h_2 - h_1 \newline \dot{q} = 0$ |
| Condenser | $\dot{q} = h_3 - h_2 \newline \dot{w}_s = 0$ |
| Compressor | $\dot{w}_c = h_4 - h_3 = v\Delta P \newline \dot{q} = 0$ |
| Boiler | $\dot{q} = h_1 - h_4 \newline \dot{w}_s = 0$ |
| Efficiency | $\eta = \dfrac{\vert\dot{w}_s\vert - \dot{w}_c}{\dot{q}_h} = \dfrac{\vert h_2 - h_1 \vert - (h_4 - h_3)}{h_1 - h_4}$ |
| Net work | $\dot{w}_{\text{net}} = \dot{q}_H - \vert\dot{q}_C\vert = \vert\dot{w}_s\vert - \dot{w}_c$ |
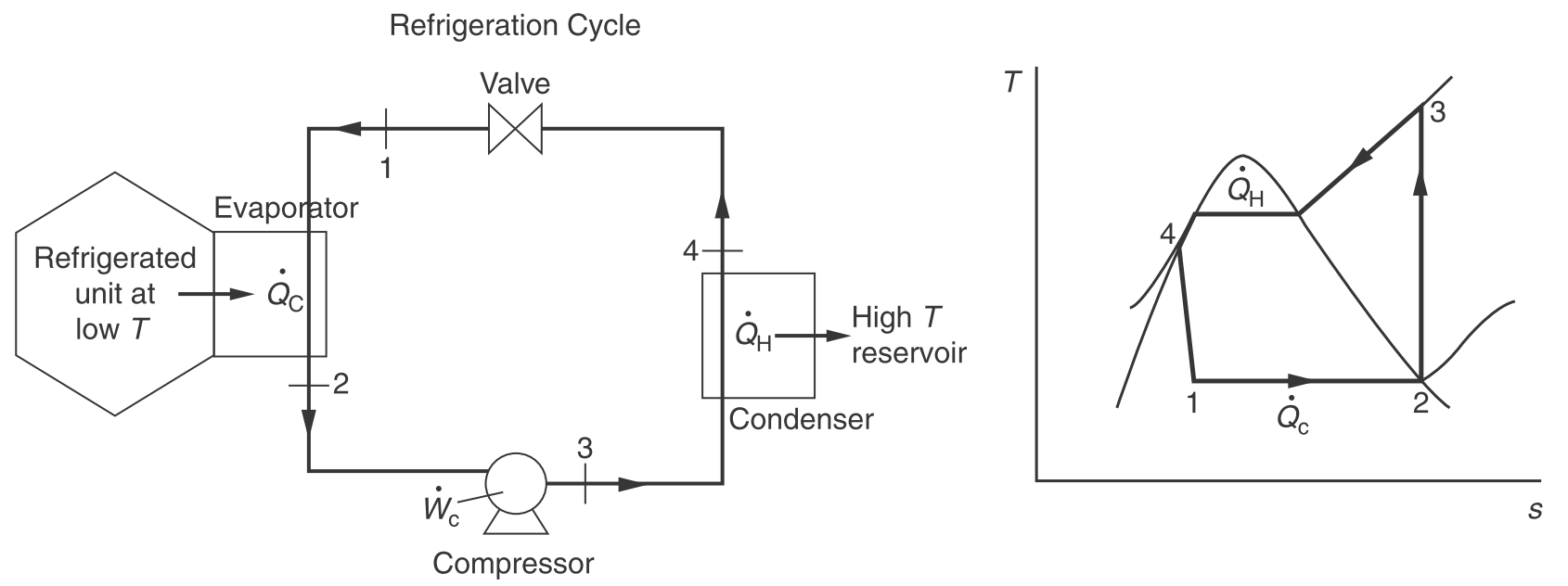
Refrigeration cycle
| Description | Equations |
|---|---|
| Evaporator | $\dot{q} = h_2 - h_1 \newline \dot{w}_s = 0$ |
| Compressor | $\dot{w}_s = h_3 - h_2 \newline \dot{q} = 0$ |
| Condenser | $\dot{q} = h_4 - h_3 \newline \dot{w}_s = 0$ |
| Value | $\dot{w}_s = 0 \newline \dot{q} = 0 \newline \Delta h = 0 \newline \Delta s > 0 \text{ (irreversible expansion)}$ |
| Coefficient of performance | $\mathrm{COP} = \dfrac{\dot{Q}_C}{\dot{W}_c} = \dfrac{h_2 - h_1}{h_3 - h_2}$ |
Intermolecular Potentials
| Description | Equations |
|---|---|
| Conservative force | $F_{ij} = -\nabla \Gamma_{ij}$ |
| Potential | $\Gamma_{ij} = -\int F_{ij} \ dr$ |
Attractive potentials (SI unit)
| Description | Equations (SI unit) |
|---|---|
| Coulomb interaction (electrostatic, point charges) |
$\Gamma_{ij}(r) = \dfrac{Q_i Q_j}{4\pi\varepsilon_0}\dfrac{1}{r}$ |
| Dipole-dipole interaction (polar, electric dipole, Keesom) |
$\Gamma_{ij}(r) = -\dfrac{(2)}{3}\dfrac{\mu_i^2 \mu_j^2}{(4\pi\varepsilon_0)^2}\dfrac{1}{kT}\dfrac{1}{r^6}$ |
| Dipole-induced dipole interaction (induction, Debye) |
$\Gamma_{ij}(r) = -\dfrac{\alpha_i \mu_j^2}{(4\pi\varepsilon_0)^2}\dfrac{1}{r^6}$ |
| Induced dipole-induced dipole interaction (dispersion, London) |
$\Gamma_{ij}(r) = -\dfrac{3}{2}\dfrac{\alpha_i \alpha_j}{(4\pi\varepsilon_0)^2}\dfrac{I_i I_j}{I_i + I_j}\dfrac{1}{r^6}$ |
| Description | Equations (SI unit) |
|---|---|
| van der Waals interaction | $\Gamma_{ij}^{\text{vdw}} = \Gamma_{ij}^{\text{K}} + \Gamma_{ij}^{\text{D}} + \Gamma_{ij}^{\text{L}} = -\dfrac{C_{\text{vdw}}}{r^6}$ |
| Keesom coefficient | $C^{\text{K}} = \dfrac{(2)}{3}\dfrac{\mu_i^2 \mu_j^2}{(4\pi\varepsilon_0)^2}\dfrac{1}{kT}$ |
| Debye coefficient | $C^{\text{D}} = \dfrac{\alpha_i \mu_j^2 + \alpha_j \mu_i^2}{(4\pi\varepsilon_0)^2}$ |
| London coefficient | $C^{\text{L}} = \dfrac{3}{2}\dfrac{\alpha_i \alpha_j}{(4\pi\varepsilon_0)^2}\dfrac{I_i I_j}{I_i + I_j}$ |
Attractive potentials (CGS unit)
| Description | Equations (CGS unit) |
|---|---|
| Coulomb interaction (electrostatic, point charges) |
$\Gamma_{ij}(r) = \dfrac{Q_i Q_j}{r}$ |
| Dipole-dipole interaction (polar, electric dipole, Keesom) |
$\Gamma_{ij}(r) = -\dfrac{(2)}{3}\dfrac{\mu_i^2 \mu_j^2}{kT}\dfrac{1}{r^6}$ |
| Dipole-induced dipole interaction (induction, Debye) |
$\Gamma_{ij}(r) = -\dfrac{\alpha_i \mu_j^2}{r^6}$ |
| Induced dipole-induced dipole interaction (dispersion, London) |
$\Gamma_{ij}(r) = -\dfrac{3}{2}\dfrac{\alpha_i \alpha_j}{r^6}\dfrac{I_i I_j}{I_i + I_j}$ |
| Description | Equations (CGS unit) |
|---|---|
| van der Waals interaction | $\Gamma_{ij}^{\text{vdw}} = \Gamma_{ij}^{\text{K}} + \Gamma_{ij}^{\text{D}} + \Gamma_{ij}^{\text{L}} = -\dfrac{C_{\text{vdw}}}{r^6}$ |
| Keesom coefficient | $C^{\text{K}} = \dfrac{(2)}{3}\dfrac{\mu_i^2 \mu_j^2}{kT}$ |
| Debye coefficient | $C^{\text{D}} = \alpha_i \mu_j^2 + \alpha_j \mu_i^2$ |
| London coefficient | $C^{\text{L}} = \dfrac{3}{2}\alpha_i \alpha_j\dfrac{I_i I_j}{I_i + I_j}$ |
Repulsive potentials
| Description | Equations (SI unit) |
|---|---|
| Hard sphere model | $\Gamma = \begin{cases} 0 & r > \sigma \\ \infty & r \le \sigma \end{cases}$ |
| Surtherland model | $\Gamma = \begin{cases} -\dfrac{C_{\text{vdw}}}{r^6} & r > \sigma \\ \infty & r \le \sigma \end{cases}$ |
| Lennard-Jones potential | $\Gamma = \dfrac{C_{\text{rep}}}{r^{12}} - \dfrac{C_{\text{vdw}}}{r^6}$ |
| Lennard-Jones potential | $\Gamma = 4\varepsilon \left[ \left(\dfrac{\sigma}{r}\right)^{12} - \left(\dfrac{\sigma}{r}\right)^6 \right]$ |
Equations of State
Principle of corresponding states
| Description | Equations |
|---|---|
| Ideal gas law | $Pv = RT$ |
| Compressibility factor | $z = \dfrac{Pv}{RT}$ |
| Reduced temperature | $T_r = \dfrac{T}{T_c}$ |
| Reduced pressure | $P_r = \dfrac{P}{P_c}$ |
| Pitzer acentric factor | $\omega = -1 - \log_{10} [P_r^{\text{sat}}(T_r = 0.7)]$ |
| Generalized compressibility | $z = z^{(0)} + \omega z^{(1)}$ |
Cubic EOS
van der Waals EOS
| Description | Equations |
|---|---|
| van der Waals EOS (pressure explicit form) |
$P = \dfrac{RT}{v-b} - \dfrac{a}{v^2}$ |
| van der Waals EOS (cubic form) |
$Pv^3 - (RT + Pb)v^2 + av - ab = 0$ |
| van der Waals EOS (reduced form) |
$P = \dfrac{8T_r}{3v_r - 1} - \dfrac{3}{v_r^2}$ |
| Intermolecular force (pressure) correction | $a = \dfrac{27}{64}\dfrac{(RT_c)^2}{P_c}$ |
| Volume correction | $b = \dfrac{RT_c}{8P_c}$ |
| Critical compressibility factor | $z_c = \frac{3}{8}$ |
Redlich-Kwong EOS
| Description | Equations |
|---|---|
| Redlich-Kwong EOS | $P = \dfrac{RT}{v-b} - \dfrac{a}{\sqrt{T}v(v+b)}$ |
| Intermolecular force (pressure) correction | $a = 0.42748 \dfrac{R^2 T_c^{2.5}}{P_c}$ |
| Volume correction | $b = 0.08664 \dfrac{RT_c}{P_c}$ |
| Critical compressibility factor | $z_c = \frac{1}{3}$ |
Peng-Robinson EOS
| Description | Equations |
|---|---|
| Peng-Robinson EOS | $P = \dfrac{RT}{v-b} - \dfrac{a \alpha(T)}{v(v+b) + b(v-b)}$ |
| Intermolecular force (pressure) correction | $a = 0.45724 \dfrac{R^2 T_c^{2}}{P_c}$ |
| Volume correction | $b = 0.07780 \dfrac{RT_c}{P_c}$ |
| Constant | $\alpha(T) = [1 + \kappa(1 - \sqrt{T_r})]^2$ |
| Constant | $\kappa = 0.37464 + 1.54226\omega - 0.26992\omega^2$ |
| Critical compressibility factor | $z_c = 0.307$ |
Virial EOS
| Description | Equations |
|---|---|
| Virial EOS | $z = \dfrac{Pv}{RT} = 1 + \dfrac{B}{v} + \dfrac{C}{v^2} + \dfrac{D}{v^3} + \cdots$ |
| Second virial coefficient | $B = \dfrac{RT_c B_r}{P_c}$ |
| Reduced second virial coefficient | $B_r = B^{(0)} + \omega B^{(1)}$ |
| 0th order correction | $B^{(0)} = 0.083 - \dfrac{0.422}{T_r^{1.6}}$ |
| 1st order correction | $B^{(1)} = 0.139 - \dfrac{0.172}{T_r^{4.2}}$ |
Mixing rules of EOS parameters
Cubic EOS
| Description | Equations |
|---|---|
| $a$ for binary mixtures | $a_{\text{mix}} = y_1^2 a_1 + 2 y_1y_2 a_{12} + y_2^2 a_2$ |
| $a$ of different species interaction | $a_{12} = \sqrt{a_1 a_2}(1 - k_{12})$ |
| $b$ for binary mixtures | $b_{\text{mix}} = y_1b_1 + y_2b_2$ |
| $a$ for multicomponent mixtures | $a_{\text{mix}} = \sum\limits_i\sum\limits_j y_i y_j a_{ij}$ |
| $b$ for multicomponent mixtures | $b_{\text{mix}} = \sum\limits_i y_i b_{i}$ |
Virial EOS
| Description | Equations |
|---|---|
| Second virial coefficient for binary mixture | $B_{\text{mix}} = y_1^2 B_{11} + 2 y_1 y_2 B_{12} + y_2^2 B_{22}$ |
| Second virial coefficient for multicomponent mixture | $B_{\text{mix}} = \sum\limits_i\sum\limits_j y_i y_j B_{ij}$ |
| Third virial coefficient for multicomponent mixture | $C_{\text{mix}} = \sum\limits_i\sum\limits_j\sum\limits_k y_i y_j y_k C_{ijk}$ |
Principle of corresponding state
| Description | Equations |
|---|---|
| Pseudocritical temperature | $T_{pc} = \sum y_i T_{c, i}$ |
| Pseudocritical pressure | $P_{pc} = \sum y_i P_{c, i}$ |
| Pseudocritical acentric factor | $\omega_{pc} = \sum y_i \omega_{c, i}$ |
EOS for liquids and solids
| Description | Equations |
|---|---|
| Thermal expansion coefficient | $\beta = \dfrac{1}{v} \left(\dfrac{\partial v}{\partial T}\right)_P$ |
| Isothermal compressibility | $\kappa = -\dfrac{1}{v} \left(\dfrac{\partial v}{\partial P}\right)_T$ |
| Rackett equation | $v_l^{\text{sat}} = \dfrac{RT_c}{P_c} (0.29056 - 0.08775 \omega)^{(1 + (1 - T_r)^{2/7})}$ |
Thermodynamic Relations
Mathematical Relations
| Description | Equations |
|---|---|
| Total differential | $dz = \left(\dfrac{\partial z}{\partial x}\right)_y dx + \left(\dfrac{\partial z}{\partial y}\right)_x dy$ |
| Clairaut’s theorem Symmetry of second derivative |
$\dfrac{\partial}{\partial x} \left(\dfrac{\partial z}{\partial y} \right) = \dfrac{\partial}{\partial y} \left( \dfrac{\partial z}{\partial x} \right)$ |
| Chain rule | $\dfrac{\partial z}{\partial x} = \dfrac{\partial z}{\partial y}\dfrac{\partial y}{\partial x}$ |
| Cyclic relation Triple chain rule |
$\left(\dfrac{\partial x}{\partial y}\right)_z \left(\dfrac{\partial y}{\partial z}\right)_x \left(\dfrac{\partial z}{\partial x}\right)_y = -1$ |
Thermodynamic Relations
| Relations | Internal energy $u$ | Enthalpy $h$ | Helmholz energy $a$ | Gibbs energy $g$ |
|---|---|---|---|---|
| Definition | - | $h = u + Pv$ | $a = u - Ts$ | $g = h - Ts$ |
| Fundamental property relations | $du = Tds - Pdv$ | $dh = Tds + vdP$ | $da = -sdT - Pdv$ | $dg = -sdT + vdP$ |
| Fundamental grouping | $\lbrace u, s, v \rbrace$ | $\lbrace h, s, P \rbrace$ | $\lbrace a, T, v \rbrace$ | $\lbrace g, T, P \rbrace$ |
| Fundamental grouping relations | $\left(\frac{\partial u}{\partial s}\right)_v = T$ | $\left(\frac{\partial h}{\partial s}\right)_P = T$ | $\left(\frac{\partial a}{\partial T}\right)_v = -s$ | $\left(\frac{\partial g}{\partial T}\right)_P = -s$ |
| Fundamental grouping relations | $\left(\frac{\partial u}{\partial v}\right)_s = -P$ | $\left(\frac{\partial h}{\partial P}\right)_s = v$ | $\left(\frac{\partial a}{\partial v}\right)_T = -P$ | $\left(\frac{\partial g}{\partial P}\right)_T = v$ |
| Maxwell’s relations | $\left(\frac{\partial T}{\partial v}\right)_s = -\left(\frac{\partial P}{\partial s}\right)_v$ | $\left(\frac{\partial T}{\partial P}\right)_s = \left(\frac{\partial v}{\partial s}\right)_P$ | $\left(\frac{\partial s}{\partial v}\right)_T = \left(\frac{\partial P}{\partial T}\right)_v$ | $\left(\frac{\partial s}{\partial P}\right)_T = -\left(\frac{\partial v}{\partial T}\right)_P$ |
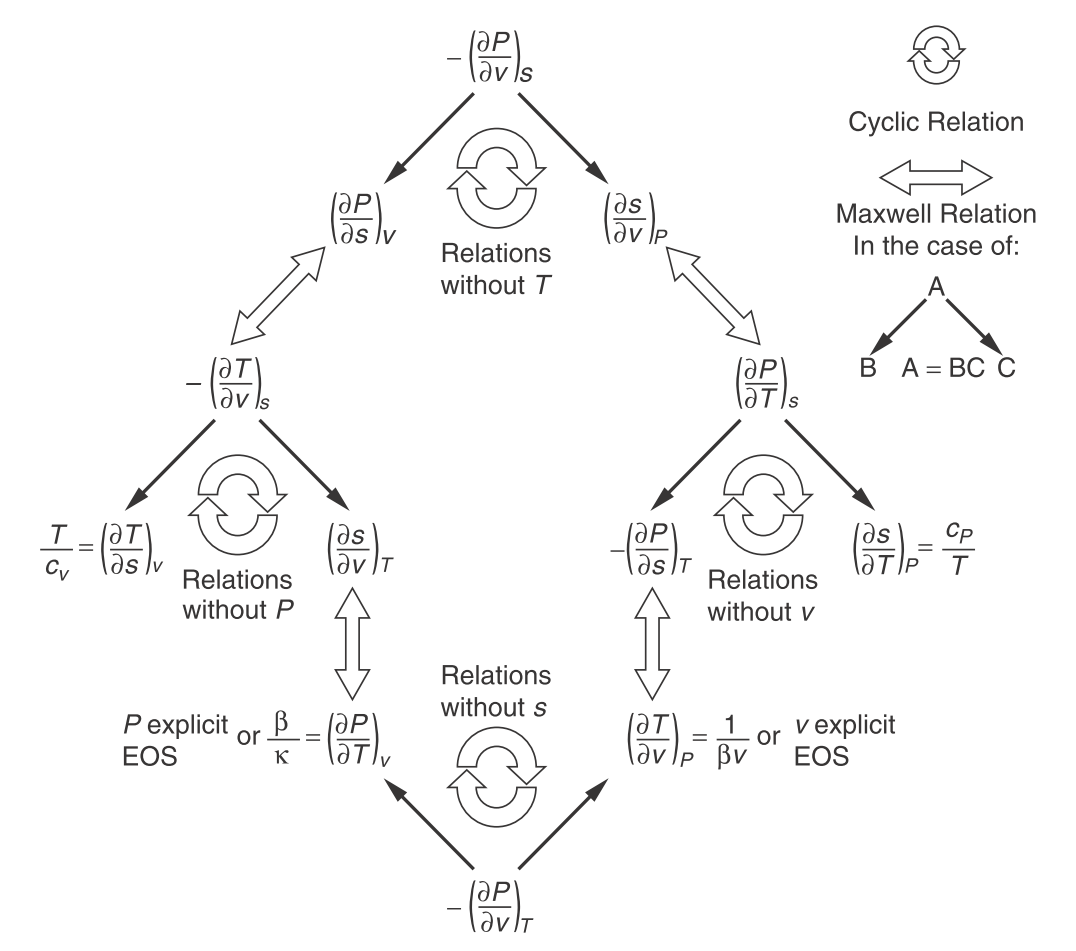
Measurable properties
| Description | Equations |
|---|---|
| Constant volume heat capacity | $c_v = \left(\dfrac{\partial u}{\partial T}\right)_v = T \left(\dfrac{\partial s}{\partial T}\right)_v$ |
| Constant pressure heat capacity | $c_P = \left(\dfrac{\partial h}{\partial T}\right)_P = T \left(\dfrac{\partial s}{\partial T}\right)_P$ |
| Constant volume heat capacity of real gas | $c_v^{\text{real}} = c_v^{\text{ideal}} + \displaystyle\int_{v_{\text{ideal}}}^{v_{\text{real}}} \left[T \left(\dfrac{\partial^2 P}{\partial T^2}\right)_v\right] dv$ |
| Constant pressure heat capacity of real gas | $c_P^{\text{real}} = c_P^{\text{ideal}} - \displaystyle\int_{P_{\text{ideal}}}^{P_{\text{real}}} \left[T \left(\dfrac{\partial^2 v}{\partial T^2}\right)_P\right] dP$ |
| Thermal expansion coefficient | $\beta = \dfrac{1}{v} \left(\dfrac{\partial v}{\partial T}\right)_P$ |
| Thermal expansion coefficient of ideal gas | $\beta = \dfrac{1}{T}$ |
| Isothermal compressibility | $\kappa = -\dfrac{1}{v} \left(\dfrac{\partial v}{\partial P}\right)_T$ |
| Isothermal compressibility of ideal gas | $\kappa = \dfrac{1}{P}$ |
Property changes
| Description | Equations |
|---|---|
| Entropy change $s(T, v)$ | $ds = \dfrac{c_v}{T} dT + \left(\dfrac{\partial P}{\partial T}\right)_v dv$ |
| Entropy change $s(T, P)$ | $ds = \dfrac{c_P}{T} dT + \left(\dfrac{\partial v}{\partial T}\right)_P dP$ |
| Internal energy change $u(T, v)$ | $du = c_v dT + \left[T \left(\dfrac{\partial P}{\partial T}\right)_v - P\right] dv$ |
| Enthalpy change $h(T, P)$ | $dh = c_P dT + \left[-T \left(\dfrac{\partial v}{\partial T}\right)_P + v\right] dP$ |
Property changes $(T, P)$
| General $f(T, P)$ | Ideal gas $\beta = \frac{1}{T}, \kappa = \frac{1}{P}$ |
|---|---|
| $ds = \dfrac{c_P}{T} dT - \beta v \ dP$ | $ds = \dfrac{c_P}{T} dT - \dfrac{R}{P} dP$ |
| $dv = \beta v \ dT - \kappa v \ dP$ | $dv = \dfrac{v}{T}dT - \dfrac{v}{P}dP$ |
| $du = (c_P - \beta Pv)dT + (\kappa Pv - \beta vT)dP$ | $du = (c_P - R)dT$ |
| $dh = (c_P - \beta Pv)dT + v \ dP$ | $dh = c_P \ dT$ |
| $da = -s \ dT + (\kappa Pv - \beta vT)dP$ | $da = -s \ dT$ |
| $dg = -s \ dT + v \ dP$ | $dg = -s \ dT + v \ dP$ |
Departure functions
| Description | Equations |
|---|---|
| General departure function | $\mathrm{dep = real - ideal}$ |
| Enthalpy departure function | $\Delta h^{\text{dep}} = h^{\text{real}} - h^{\text{ideal}}$ |
| Entropy departure function | $\Delta s^{\text{dep}} = s^{\text{real}} - s^{\text{ideal}}$ |
| Dimensionless enthalpy departure function | $\dfrac{\Delta h^{\text{dep}}}{RT_c} = T_r^2 \displaystyle\int_0^P \left[-\dfrac{1}{P_r}\left(\dfrac{\partial z}{\partial T_r}\right)_P \right] dP_r$ |
| Dimensionless entropy departure function | $\dfrac{\Delta s^{\text{dep}}}{R} = \displaystyle\int_0^P -\left[\dfrac{z-1}{P_r} + \dfrac{T_r}{P_r}\left(\dfrac{\partial z}{\partial T_r}\right)_P \right] dP_r$ |
| Dimensionless enthalpy departure function with Lee-Kesler EOS | $\dfrac{\Delta h^{\text{dep}}}{RT_c} = \left[\dfrac{\Delta h^{\text{dep}}}{RT_c}\right]^{(0)} + \omega \left[\dfrac{\Delta h^{\text{dep}}}{RT_c}\right]^{(1)}$ |
| Dimensionless entropy departure function with Lee-Kesler EOS | $\dfrac{\Delta s^{\text{dep}}}{R} = \left[\dfrac{\Delta s^{\text{dep}}}{R}\right]^{(0)} + \omega \left[\dfrac{\Delta s^{\text{dep}}}{R}\right]^{(1)}$ |
Joule-Thomson Expansion
| Description | Equations |
|---|---|
| Joule-Thomson expansion Adiabatic reversible throttling |
$\dot{q} = 0 \newline \dot{w}_s = 0 \newline \Delta h = 0$ |
| Joule-Thomson coefficient | $\mu_{\text{JT}} = \left(\dfrac{\partial T}{\partial P}\right)_h$ |
| Joule-Thomson coefficient | $\mu_{\text{JT}} = \dfrac{\left[-T \left(\dfrac{\partial v}{\partial T}\right)_P + v\right]}{c_P^{\text{real}}}$ |
Phase Equilibria
Single-component equilibrium
| Description | Equations |
|---|---|
| Gibbs free energy | $g = h - Ts$ |
| Second law of thermodynamics | $dG_i \le 0$ |
| Criteria for chemical equilibrium | $g_i^\alpha = g_i^\beta$ |
| Clapeyron equation General phase equilibrium |
$\dfrac{dP}{dT} = \dfrac{\Delta s}{\Delta v} = \dfrac{\Delta h}{T\Delta v}$ |
| Clausius-Clapeyron equation ★ Vapor-liquid equilibrium ★ Ideal gas, negligible liquid volume |
$\dfrac{dP^{\text{sat}}}{P^{\text{sat}}} = \dfrac{\Delta h_{\text{vap}} dT}{RT^2}$ |
| Clausius-Clapeyron equation ★ Vapor-liquid equilibrium ★ Ideal gas, negligible liquid volume ★ $\Delta h_{\text{vap}}$ independent of $T$ |
$\ln\dfrac{P_2^{\text{sat}}}{P_1^{\text{sat}}} = -\dfrac{\Delta h_{\text{vap}}}{R} \left(\dfrac{1}{T_2} - \dfrac{1}{T_1}\right)$ |
| Antoine’s equation | $\ln P^{\text{sat}} = A - \dfrac{B}{C+T}$ |
Properties of mixtures
| Description | Equations |
|---|---|
| Extensive total solution (mixture) property | $K$ |
| Intensive total solution (mixture) property | $k = \dfrac{K}{n}$ |
| Extensive pure species property | $K_i$ |
| Intensive pure species property | $k_i = \dfrac{K_i}{n_i}$ |
| Partial molar property | $\overline{K}_i = \left(\dfrac{\partial K}{\partial n_i}\right)_{T, P, n_{j\not= i}}$ |
| Limiting case of partial molar property | $\displaystyle\lim_{x_i \to 1} \overline{K}_i = k_i \newline \lim_{x_i \to 0} \overline{K}_i = \overline{K}_i^\infty$ |
| Differential of extensive property | $dK = \left(\frac{\partial K}{\partial T}\right)_{P, n_i} dT + \left(\frac{\partial K}{\partial P}\right)_{T, n_i} dP + \sum \overline{K}_i dn_i$ |
| Relation between properties ★ Constant T, P |
$K = \sum n_i \overline{K}_i \newline k = \sum x_i \overline{K}_i$ |
| Gibbs-Duhem equation ★ Constant T, P |
$\sum n_i d\overline{K}_i = 0$ |
| Corollary of Gibbs-Duhem equation ★ Binary mixture |
$x_1 \dfrac{d\overline{K_1}}{d x_1} + x_2 \dfrac{d\overline{K_2}}{d x_1} = 0$ |
Property changes of mixtures
| Description | Equations |
|---|---|
| Extensive property change of mixing | $\Delta K_{\text{mix}} = K - \sum n_i k_i \newline \Delta K_{\text{mix}} = \sum n_i (\overline{K}_i - k_i)$ |
| Intensive property change of mixing | $\Delta k_{\text{mix}} = k - \sum x_i k_i \newline \Delta k_{\text{mix}} = \sum x_i (\overline{K}_i - k_i)$ |
| Enthalpy of mixing | $\Delta h_{\text{mix}} = \sum x_i (\overline{h}_i - h_i)$ |
| Enthalpy of mixing | $\Delta h_{\text{mix}} = \dfrac{\Delta \tilde{h}_s}{n+1} = \Delta \tilde{h}_s x_1$ |
| Enthalpy of solution | $\Delta \tilde{h}_s = \dfrac{\Delta h_{\text{mix}}}{x_1} = \Delta h_{\text{mix}}(n+1)$ |
| Entropy of mixing ★ Ideal gas, regular solution |
$\Delta s_{\text{mix}} = -R\sum y_i \ln y_i$ |
| Partial molar property change of mixing | $\overline{\Delta K}_{\text{mix}, i} = \overline{K}_i - k_i$ |
Determination of $\overline{G}_i$
| Description | Equations |
|---|---|
| Partial molar volume of species 1 ★ Virial EOS |
$\overline{V}_1 = \dfrac{RT}{P} + (y_1^2 + 2y_1 y_2)B_{11} + 2y_2^2 B_{12} - y_2^2 B_{22}$ |
| Partial molar volume of species 2 ★ Virial EOS |
$\overline{V}_2 = \dfrac{RT}{P} - y_1^2 B_{11} + 2y_1^2 B_{12} + (y_2^2 + 2y_1 y_2)B_{22}$ |
| Volume change of mixing ★ Virial EOS |
$\Delta v_{\text{mix}} = y_1 y_2(2B_{12} - B_{11} - B_{22})$ |
| Partial molar property | $\overline{K}_i = k_i + \overline{\Delta K}_{\text{mix}, i}$ |
| Graphical method Slope is difference |
$\dfrac{dk}{dx_1} = \overline{K}_1 - \overline{K}_2$ |
| Graphical method $\overline{K}_2$ is intercept |
$k = x_1 \dfrac{dk}{dx_1} + \overline{K}_2$ |
| Graphical method $\overline{K}_2$ explicit |
$\overline{K}_2 = k - x_1 \dfrac{dk}{dx_1}$ |
$T$ and $P$ dependence of $\overline{G}_i$
| Description | Equations |
|---|---|
| Partial molar Gibbs energy dependence on temperature | $\left(\dfrac{\partial \overline{G}_i}{\partial T}\right)_{P, n_i} = -\overline{S}_i$ |
| Partial molar Gibbs energy dependence on temperature (measurable) | $\left[\dfrac{\partial}{\partial T}\left(\dfrac{\overline{G}_i}{T}\right)\right]_{P, n_i} = -\dfrac{\overline{H}_i}{T^2}$ |
| Partial molar Gibbs energy dependence on pressure | $\left(\dfrac{\partial \overline{G}_i}{\partial P}\right)_{T, n_i} = -\overline{V}_i$ |
Multicomponent equilibrium
| Description | Equations |
|---|---|
| Chemical potential | $\mu_i = \overline{G}_i = \left(\dfrac{\partial G}{\partial n_i}\right)_{T, P, n_{j \not= i}}$ |
| Criteria for chemical equilibrium | $\mu_i^\alpha = \mu_i^\beta$ |
| General multicomponent equilibrium | $\Delta \left[ -\dfrac{\overline{H}_i}{T^2}dT - \dfrac{\overline{V}_i}{T}dP + \dfrac{1}{T} \left[\dfrac{\partial \mu_i}{\partial x_i}\right]_{T, P} dx_i \right] = 0$ |
| Vapor liquid equilibrium ★ Ideal gas |
$\begin{aligned} &-\dfrac{h_i^v}{T^2}dT - R\dfrac{dP}{P} + R\dfrac{dx_i^v}{x_i^v} = -\dfrac{\overline{H}_i^l}{T^2}dT - \dfrac{\overline{V}_i^l}{T}dP + \dfrac{1}{T} \left[\dfrac{\partial \mu_i^l}{\partial x_i}\right]_{T, P} dx_i \end{aligned}$ |
Fugacity
Definition of fugacity
| Description | Equations |
|---|---|
| Definition of fugacity of pure species ★ Constant T |
$g_i - g_i^\circ = RT\ln\left(\dfrac{f_i}{f_i^\circ}\right) \newline \lim\limits_{P \to 0} \varphi_i = 1$ |
| Fugacity of pure species ★ Constant T |
$f_i = \varphi_i P$ |
| Fugacity coefficient of pure species | $\varphi_i = \dfrac{f_i}{P}$ |
| Definition of fugacity of species i in mixture | $\mu_i - \mu_i^\circ = RT\ln\left(\dfrac{\hat{f}_i}{\hat{f}_i^\circ}\right) \newline \lim\limits_{P \to 0} \hat{\varphi}_i = 1$ |
| Fugacity of species i in mixture | $\hat{f}_i = y_i P \hat{\varphi}_i$ |
| Fugacity coefficient of species i in mixture ★ Constant T |
$\hat{\varphi}_i = \dfrac{\hat{f}_i}{p_i} = \dfrac{\hat{f}_i}{y_i P}$ |
| Criteria for chemical equilibrium | $\hat{f}_i^\alpha = \hat{f}_i^\beta$ |
Fugacity in vapor phase
Single component pure species
| Description | Equations |
|---|---|
| Reference state ★ Ideal gas |
$P^\circ = P_{\text{low}} \newline T^\circ = T_{\text{sys}} \newline \hat{f}_i^\circ = P^\circ$ |
| Fugacity of pure species ★ Constant T |
$f_i = \varphi_i P$ |
| Fugacity coefficient of pure species | $\varphi_i = \dfrac{f_i}{P}$ |
| Fugacity from experimental data | $f_i^v = P^\circ \exp\left(\dfrac{g_i - g_i^\circ}{RT}\right)$ |
| Fugacity coefficient from experimental data | $\varphi_i = \dfrac{P^\circ}{P} \exp\left(\dfrac{g_i - g_i^\circ}{RT}\right)$ |
| Fugacity from EOS | $RT\ln\left(\dfrac{f_i^v}{P^\circ}\right) = \displaystyle\int_{P^\circ}^P v_i \ dP$ |
| Fugacity coefficient from EOS | $\varphi_i = \dfrac{P^\circ}{P} \exp\left[\dfrac{1}{RT}\displaystyle\int_{P^\circ}^P v_i \ dP\right]$ |
| Fugacity coefficient from vdW EOS | $\ln\varphi_i^v = -\ln\left[\dfrac{(v_i - b)P}{RT}\right] + \dfrac{b}{v_i - b} - \dfrac{2a}{RTv_i}$ |
| Fugacity coefficient from virial form of vdW EOS | $\ln\varphi_i^v = \left(b - \dfrac{a}{RT}\right) \dfrac{P}{RT}$ |
| Fugacity coefficient from generalized correlations | $\ln \varphi_i^v = \displaystyle\int_{P^\circ}^P (z_i - 1) \dfrac{dP}{P}$ |
| Generalized fugacity coefficient with Lee-Kesler EOS | $\log\varphi_i = \log\varphi^{(0)} + \omega \log\varphi^{(1)}$ |
Multicomponent mixtures
| Description | Equations |
|---|---|
| Reference state ★ Ideal gas |
$P^\circ = P_{\text{low}} \newline T^\circ = T_{\text{sys}} \newline n_i^\circ = n_{i, \text{sys}} \newline f_i^\circ = y_i P^\circ \newline V^\circ = \dfrac{nRT}{P^\circ}$ |
| Fugacity of species i in mixture ★ EOS ★ Full i-j interaction |
$\hat{f}_i = y_i \hat{\varphi}_i P$ |
| Fugacity of species i in mixture ★ Lewis fugacity rule ★ Same species interaction only, i-i interaction |
$\hat{f}_i = y_i \varphi_i P \newline \hat{\varphi}_i = \varphi_i$ |
| Fugacity of species i in mixture ★ Ideal gas, no interaction |
$\hat{f}_i = y_i P \newline \hat{\varphi}_i = 1$ |
| Fugacity coefficient of species i in mixture ★ Constant T |
$\hat{\varphi}_i = \dfrac{\hat{f}_i}{p_i} = \dfrac{\hat{f}_i}{y_i P}$ |
| Fugacity coefficient from v-explicit EOS | $\hat{\varphi}_i = \dfrac{P^\circ}{P} \exp\left[\dfrac{1}{RT}\displaystyle\int_{P^\circ}^P \overline{V}_i \ dP\right]$ |
| Fugacity coefficient from P-explicit EOS | $\hat{\varphi}_i = \dfrac{P^\circ}{P} \exp\left[-\dfrac{1}{RT}\displaystyle\int_{V^\circ}^V \overline{P}_i \ dV\right]$ |
Fugacity coefficients from cubic EOS
van der Waals EOS
| Description | van der Waals EOS |
|---|---|
| Pure species i | $\ln \varphi_{i}=\dfrac{b_{i}}{v_{i}-b_{i}}-\ln \left(\dfrac{\left(v_{i}-b_{i}\right) P}{R T}\right)-\dfrac{2 a_{i}}{R T v_{i}}$ |
| Species 1 in a binary mixture | $\ln \hat{\varphi}_{1}=\dfrac{b_{1}}{v-b}-\ln \left(\dfrac{(v-b) P}{R T}\right)-\dfrac{2\left(y_{1} a_{1}+y_{2} a_{12}\right)}{R T v}$ |
| Species i in a mixture | $\ln \hat{\varphi}_{i}=\dfrac{b_{i}}{v-b}-\ln \left(\dfrac{(v-b) P}{R T}\right)-\dfrac{2 \sum\limits_{k=1}^{m} y_{k} a_{i k}}{R T v}$ |
Redlich-Kwong EOS
| Description | Redlich-Kwong EOS |
|---|---|
| Pure species i | $\begin{aligned}\ln \varphi_{i} =& z_{i}-1-\ln \left(\dfrac{\left(v_{i}-b_{i}\right) P}{R T}\right) \\ &- \dfrac{a_{i}}{b_{i} R T^{1.5}} \ln \left(1+\dfrac{b_{i}}{v_{i}}\right)\end{aligned}$ |
| Species 1 in a binary mixture | $\begin{aligned}\ln \hat{\varphi}_{1} =& \dfrac{b_{1}}{b}(z-1)-\ln \left(\dfrac{(v-b) P}{R T}\right) \\ &+ \dfrac{1}{b R T^{1.5}}\left[\dfrac{a b_{1}}{b}-2\left(y_{1} a_{1}+y_{2} a_{12}\right)\right] \ln \left(1+\dfrac{b}{v}\right)\end{aligned}$ |
| Species i in a mixture | $\begin{aligned}\ln \hat{\varphi}_{i} =& \dfrac{b_{i}}{b}(z-1)-\ln \left(\dfrac{(v-b) P}{R T}\right) \\ &+ \dfrac{1}{b R T^{1.5}}\left[\dfrac{a b_{i}}{b}-2 \sum\limits_{k=1}^{m} y_{k} a_{i k}\right] \ln \left(1+\dfrac{b}{v}\right)\end{aligned}$ |
Peng-Robinson EOS
| Description | Peng-Robinson EOS |
|---|---|
| Pure species i | $\ln \varphi_{i}= z_{i}-1-\ln \left(\dfrac{\left(v_{i}-b_{i}\right) P}{R T}\right)-\dfrac{(a \alpha)_{i}}{2 \sqrt{2} b_{i} R T} \ln \left[\dfrac{v_{i}+(1+\sqrt{2}) b_{i}}{v_{i}+(1-\sqrt{2}) b_{i}}\right]$ |
| Species 1 in a binary mixture | $\begin{aligned}\ln \hat{\varphi}_{1} =& \dfrac{b_{1}}{b}(z-1)-\ln \left(\dfrac{(v-b) P}{R T}\right) \\ &+ \dfrac{a \alpha}{2 \sqrt{2} b R T}\left[\dfrac{b_{1}}{b}-\dfrac{2}{a \alpha}\left(y_{1}(a \alpha)_{1}+y_{2}(a \alpha)_{12}\right)\right] \ln \left[\dfrac{v+(1+\sqrt{2}) b}{v+(1-\sqrt{2}) b}\right]\end{aligned}$ |
| Species i in a mixture | $\begin{aligned}\ln \hat{\varphi}_{i} =& \dfrac{b_{i}}{b}(z-1)-\ln \left(\dfrac{(v-b) P}{R T}\right) \\ &+ \dfrac{a \alpha}{2 \sqrt{2} b R T}\left[\dfrac{b_{i}}{b}-\dfrac{2}{a \alpha} \sum\limits_{k=1}^{m} y_{k}(a \alpha)_{i k}\right] \ln \left[\dfrac{v+(1+\sqrt{2}) b}{v+(1-\sqrt{2}) b}\right]\end{aligned}$ |
Mixing rules
| Description | Equations |
|---|---|
| Interaction parameter $a$ for multicomponent mixtures | $a_{\text{mix}} = \sum\sum y_i y_j a_{ij}$ |
| Like attractions | $a_{ii} = a_i$ |
| Unlike attractions | $a_{ij} = \sqrt{a_{ii}a_{jj}}(1 - k_{ij})$ |
| Volume parameter $a$ for multicomponent mixtures | $b_{\text{mix}} = \sum y_i b_{i}$ |
Property changes of mixing of ideal gas
| Description | Equations |
|---|---|
| Volume change of mixing | $\Delta v_{\text{mix}} = 0$ |
| Enthalpy change of mixing | $\Delta h_{\text{mix}} = 0$ |
| Entropy change of mixing | $\Delta s_{\text{mix}} = -R\sum y_i \ln y_i > 0$ |
| Gibbs energy change of mixing | $\Delta g_{\text{mix}} = RT \sum y_i \ln y_i < 0$ |
Fugacity in liquid phase
Reference states
| Description | Equations |
|---|---|
| Reference state of fugacity in ideal solution | $\hat{f}_i^\circ = x_i f_i^\circ$ |
| Lewis/Randall rule reference state of fugacity ★ Solvent, pure limit ★ Same species (a-a) interaction only |
$f_i^\circ = f_i \newline \hat{f}_i^\circ = x_i f_i$ |
| Henry’s law reference state of fugacity ★ Solute, dilute limit ★ Different species (a-b) interaction only |
$f_i^\circ = \mathcal{H}_i \newline \hat{f}_i^\circ = x_i \mathcal{H}_i$ |
Lewis/Randall rule
| Description | Equations |
|---|---|
| Lewis/Randall rule reference state of fugacity ★ Solvent, pure limit ★ Same species (a-a) interaction only |
$f_i^\circ = f_i \newline \hat{f}_i^\circ = x_i f_i$ |
| Pure liquid fugacity with Poynting correction at T, P | $f_i^l = \varphi_i^{\text{sat}} P_i^{\text{sat}} \exp\left[ \displaystyle\int_{P_i^{\text{sat}}}^{P} \dfrac{v_i^l}{RT} dP \right]$ |
| Pure liquid fugacity with Poynting correction at T, P ★ Incompressible liquid |
$f_i^l = \varphi_i^{\text{sat}} P_i^{\text{sat}} \exp\left[ \dfrac{v_i^l}{RT} (P - P_i^{\text{sat}}) \right]$ |
| Pure liquid fugacity ★ $P \approx P^{\text{sat}}$ |
$f_i^l = \varphi_i^{\text{sat}} P_i^{\text{sat}}$ |
| Pure liquid fugacity ★ Ideal gas (low P, low sat P) |
$f_i^l = P_i^{\text{sat}}$ |
Henry’s law
| Description | Equations |
|---|---|
| Henry’s law reference state of fugacity ★ Solute, dilute limit ★ Different species (a-b) interaction only |
$f_i^\circ = \mathcal{H}_i \newline \hat{f}_i^\circ = x_i \mathcal{H}_i$ |
| Pressure dependence of Henry’s constant | $\mathcal{H}_i(P) = \mathcal{H}_i(P_1) \exp\left[ \displaystyle\int_{P_0}^{P} \dfrac{\overline{V}_i^\infty}{RT} dP \right]$ |
| Temperature dependence of Henry’s constant | $\mathcal{H}_i(T) = \mathcal{H}_i(T_1) \exp\left[ \displaystyle\int_{T_0}^{T} \dfrac{h_i^v - \overline{H}_i^\infty}{RT^2} dP \right]$ |
Activity coefficient
| Description | Equations |
|---|---|
| Activity coefficient | $\gamma_i = \dfrac{\hat{f}_i^l}{\hat{f}_i^\circ} = \dfrac{\hat{f}_i^l}{x_i f_i^\circ}$ |
| Activity coefficient in Lewis/Randall rule reference state | $\lim\limits_{x_i \to 0} \gamma_i^{\text{LR}} = \dfrac{\mathcal{H}_i}{f_i} \newline \lim\limits_{x_i \to 1} \gamma_i^{\text{LR}} = 1$ |
| Activity coefficient in Henry’s law reference state | $\lim\limits_{x_i \to 0} \gamma_i^{\text{H}} = 1 \newline \lim\limits_{x_i \to 1} \gamma_i^{\text{H}} = \dfrac{f_i}{\mathcal{H}_i}$ |
| Activity | $a_i = \dfrac{\hat{f}_i^l}{f_i^\circ} \newline a_i = x_i \gamma_i$ |
| Gibbs-Duhem Equation ★ Constant T, P |
$\sum x_i d(\ln \gamma_i) = 0$ |
| Corollary of Gibbs-Duhem equation ★ Binary mixture |
$x_1 \left(\dfrac{\partial \ln \gamma_1}{\partial x_1}\right)_{T, P} + x_2 \left(\dfrac{\partial \ln \gamma_2}{\partial x_2}\right)_{T, P} = 0$ |
Excess properties
| Description | Equations |
|---|---|
| Excess property | $k^E = k^{\text{real}} - k^{\text{ideal}}$ |
| Excess property | $k^E = \Delta k_{\text{mix}}^{\text{real}} - \Delta k_{\text{mix}}^{\text{ideal}}$ |
| Partial molar excess property | $\overline{K}_i^E = \overline{K}_i^{\text{real}} - \overline{K}_i^{\text{ideal}}$ |
| Excess Gibbs free energy | $g^E = \Delta g_{\text{mix}} - RT \sum x_i \ln x_i$ |
| Excess Gibbs free energy | $g^E = RT \sum x_i \ln \gamma_i$ |
| Partial molar excess Gibbs free energy | $\overline{G}_i^E = RT \ln \gamma_i$ |
| Area test for thermodynamic consistency ★ Lewis/Randall reference state ★ Constant T, P |
$\displaystyle\int_0^1 \ln \left(\dfrac{\gamma_a}{\gamma_b}\right) dx_a = 0$ |
$\gamma_i$ for binary systems
Two-suffix Margules equation
| Description | Equations |
|---|---|
| Two-suffix Margules equation | $g^E = A x_a x_b$ |
| Activity coefficient | $\overline{G}_i^{E} = RT\ln\gamma_i \newline \overline{G}_a^{E} = A x_b^2 \newline \overline{G}_b^{E} = A x_a^2$ |
Three-suffix Margules equation
| Description | Equations |
|---|---|
| Three-suffix Margules equation | $g^E = x_{a} x_{b}[A+B(x_{a}-x_{b})]$ |
| Activity coefficient | $\overline{G}_i^{E} = RT\ln\gamma_i \newline \overline{G}_a^{E} = (A+3 B) x_{b}^{2}-4 B x_{b}^{3} \newline \overline{G}_b^{E} = (A-3 B) x_{a}^{2}+4 B x_{a}^{3}$ |
| Three-suffix Margules equation | $g^E = x_{a} x_{b}(A_{ba} x_{a}+A_{ab} x_{b})$ |
| Activity coefficient | $\overline{G}_i^{E} = RT\ln\gamma_i \newline \overline{G}_a^{E} = x_{b}^{2}\left[A_{a b}+2\left(A_{b a}-A_{ab}\right) x_{a}\right] \newline \overline{G}_b^{E} = x_{a}^{2}\left[A_{b a}+2\left(A_{a b}-A_{b a}\right) x_{b}\right]$ |
van Laar equation
| Description | Equations |
|---|---|
| van Laar equation | $g^E = x_{a} x_{b}\left(\dfrac{A B}{A x_{a}+B x_{b}}\right)$ |
| Activity coefficient | $\overline{G}_i^{E} = RT\ln\gamma_i \newline \overline{G}_a^{E} = A\left(\dfrac{B x_{b}}{A x_{a}+B x_{b}}\right)^{2} \newline \overline{G}_b^{E} = B\left(\dfrac{A x_{a}}{A x_{a}+B x_{b}}\right)^{2}$ |
Wilson equation
| Description | Equations |
|---|---|
| Wilson equation | $g^E = -R T\left[x_{a} \ln \left(x_{a}+\Lambda_{a b} x_{b}\right) + x_{b} \ln \left(x_{b}+\Lambda_{b a} x_{a}\right)\right]$ |
| Activity coefficient | $\begin{aligned} \overline{G}_i^{E} &= RT\ln\gamma_i \\ \overline{G}_a^{E} &= -R T\left[\ln \left(x_{a}+\Lambda_{ab} x_{b}\right)+x_{b}\left(\dfrac{\Lambda_{b a}}{x_{b}+\Lambda_{ba}x_{a}}-\dfrac{\Lambda_{a b}}{x_{a}+\Lambda_{a b} x_{b}}\right)\right] \\ \overline{G}_b^{E} &= -R T\left[\ln \left(x_{b}+\Lambda_{ba}x_{a}\right)+x_{a}\left(\dfrac{\Lambda_{a b}}{x_{a}+\Lambda_{a b} x_{b}}-\dfrac{\Lambda_{b a}}{x_{b}+\Lambda_{ba}x_{a}}\right)\right] \end{aligned}$ |
| Wilson parameters | $\Lambda_{ab} = \dfrac{v_b}{v_a}\exp\left(-\dfrac{\lambda_{ab}}{RT}\right) \newline \Lambda_{ba} = \dfrac{v_a}{v_b}\exp\left(-\dfrac{\lambda_{ba}}{RT}\right)$ |
Non-random two-liquid model (NRTL)
| Description | Equations |
|---|---|
| Non-random two-liquid model (NRTL) | $g^E = R T x_{a} x_{b}\left[\dfrac{\tau_{b a} \mathbf{G}_{b a}}{x_{a}+x_{b} \mathbf{G}_{b a}}+\dfrac{\tau_{a b} \mathbf{G}_{a b}}{x_{b}+x_{a} \mathbf{G}_{a b}}\right]$ |
| Activity coefficient | $\overline{G}_i^{E} = RT\ln\gamma_i \newline \overline{G}_a^{E} = R T x_{b}^{2}\left[\dfrac{\tau_{b a} \mathbf{G}_{b a}^{2}}{\left(x_{a}+x_{b} \mathbf{G}_{b a}\right)^{2}}+\dfrac{\tau_{a b} \mathbf{G}_{a b}}{\left(x_{b}+x_{a} \mathbf{G}_{a b}\right)^{2}}\right] \newline \overline{G}_b^{E} = R T x_{a}^{2}\left[\dfrac{\tau_{b a} \mathbf{G}_{b a}}{\left(x_{a}+x_{b} \mathbf{G}_{b a}\right)^{2}}+\dfrac{\tau_{a b} \mathbf{G}_{a b}^{2}}{\left(x_{a}+x_{b} \mathbf{G}_{a b}\right)^{2}}\right]$ |
| NRTL parameters | $\mathbf{G}_{ab} = \exp(-\alpha \tau_{ab}) \newline \mathbf{G}_{ba} = \exp(-\alpha \tau_{ba})$ |
$\gamma_i$ for multicomponent systems
Two-suffix Margules equation
| Description | Equations |
|---|---|
| Two-suffix Margules equation (ternary system) | $g^E = A_{ab}x_a x_b + A_{ac}x_a x_c + A_{bc}x_b x_c$ |
| Partial excess Gibbs energy of species a | $\overline{G}^E_a = A_{ab}x_b^2 + A_{ac}x_c^2 + (A_{ab} + A_{ac} - A_{bc})x_b x_c$ |
| Partial excess Gibbs energy of species b | $\overline{G}^E_b = A_{ab}x_a^2 + A_{bc}x_c^2 + (A_{ab} + A_{bc} - A_{ac})x_a x_c$ |
| Partial excess Gibbs energy of species c | $\overline{G}^E_c = A_{ac}x_a^2 + A_{bc}x_b^2 + (A_{ac} + A_{bc} - A_{ab})x_a x_b$ |
| Two-suffix Margules equation (multicomponent system) | $g^E = \sum\limits_i \sum\limits_j \dfrac{A_{ij}}{2}x_i x_j$ |
| Two-suffix Margules parameter | $A_{ii} = 0 \newline A_{ij} = A_{ji}$ |
Wilson equation
| Description | Equations |
|---|---|
| Wilson equation | $\begin{aligned}\ln \gamma_i = 1-\ln \left(\sum\limits_{j=1}^{m} x_{j} \Lambda_{i j}\right)-\sum\limits_{k=1}^{m} \dfrac{x_{k} \Lambda_{k i}}{\ln \left(\sum\limits_{j=1}^{m} x_{j} \Lambda_{k j}\right)}\end{aligned}$ |
| Wilson parameter | $\Lambda_{jj} = 1$ |
Non-random two-liquid model (NRTL)
| Description | Equations |
|---|---|
| Non-random two-liquid model (NRTL) | $\begin{aligned}\ln \gamma_i = \frac{\sum\limits_{j=1}^{m} \tau_{j i} x_{j} \mathbf{G}_{j i}}{\sum\limits_{l=1}^{m} x_{l} \mathbf{G}_{l i}}+\sum\limits_{j=1}^{m} \frac{x_{j} \mathbf{G}_{i j}}{\sum\limits_{l=1}^{m} x_{l} \mathbf{G}_{l j}}\left(\tau_{i j}-\frac{\sum\limits_{k=1}^{m} \tau_{k j} x_{k} \mathbf{G}_{k j}}{\sum\limits_{l=1}^{m} x_{l} \mathbf{G}_{l j}}\right) \end{aligned}$ |
| NRTL parameters | $\ln \mathbf{G}_{i j}=-\alpha_{i j} \tau_{i j} \newline \tau_{i j}=0 \newline \mathbf{G}_{i j}=1$ |
Universal quasi-chemical theory (UNIQUAC)
| Description | Equations |
|---|---|
| Universal quasi-chemical theory (UNIQUAC) | $\begin{aligned} \ln\gamma_i = \ln \frac{\Phi_{i}^{*}}{x_{i}}+\frac{z}{2} q_{i} \ln \frac{\theta_{i}}{\Phi_{i}^{*}}+l_{i}+\frac{\Phi_{i}^{*}}{x_{i}} \sum\limits_{j=1}^{m} x_{j} l_{j}+q_{i}^{\prime}\left[1-\sum\limits_{j=1}^{m} \theta_{j}^{\prime} \tau_{j i}-\sum\limits_{j=1}^{m} \frac{\theta_{j}^{\prime} \tau_{i j}}{\sum\limits_{k=1}^{m} \theta_{k}^{\prime} \tau_{k j}}\right] \end{aligned}$ |
| UNIQUAC parameters | $l_{i}=\dfrac{z}{2}\left(r_{i}-q_{i}\right)-\left(r_{i}-1\right) \newline \tau_{j k}=\exp \left(-\dfrac{a_{j k}}{T}\right) \newline \tau_{k k}=1 \newline \Phi_{i}^{*}=\dfrac{x_{i} r_{i}}{\sum\limits_{j=1}^{m} x_{j} r_{j}} \newline \theta_{i}=\dfrac{x_{i} q_{i}}{\sum\limits_{j=1}^{m} x_{j} q_{j}} \newline \theta_{i}^{\prime}=\dfrac{x_{i} q_{i}^{\prime}}{\sum\limits_{j=1}^{m} x_{j} q_{j}^{\prime}}$ |
$T$ and $P$ dependence of $g^E$
| Description | Equations |
|---|---|
| Excess Gibbs energy dependence on pressure | $\left(\dfrac{\partial g^E}{\partial P}\right)_{T, n_i} = v^E = \Delta v_{\text{mix}}$ |
| Excess Gibbs energy dependence on temperature | $\left[\dfrac{\partial}{\partial T}\left(\dfrac{g^E}{T}\right)\right]_{P, n_i} = -\dfrac{h^E}{T^2} = -\dfrac{\Delta h_{\text{mix}}}{T^2}$ |
| Excess Gibbs energy dependence on temperature ★ Regular solution |
$g^E = RT\sum x_i \ln \gamma_i = \text{constant}$ |
| Excess Gibbs energy dependence on temperature ★ Athermal solution |
$\dfrac{g^E}{T} = R\sum x_i \ln \gamma_i = \text{constant}$ |
$T$ and $P$ dependence of $\gamma_i$
| Description | Equations |
|---|---|
| Activity coefficient dependence on pressure | $\left(\dfrac{\partial \ln\gamma_i}{\partial P}\right)_{T, x} = \dfrac{\overline{V}_i - v_i}{RT}$ |
| Activity coefficient dependence on temperature | $\left(\dfrac{\partial \ln\gamma_i}{\partial T}\right)_{P, x} = -\dfrac{\overline{H}_i - h_i}{RT^2}$ |
Fugacity in solid phase
| Description | Equations |
|---|---|
| Activity coefficient of pure solids | $\Gamma_i = 1$ |
| Fugacity of pure solids | $\hat{f}_i^s = f_i^s$ |
| Fugacity of solid solutions ★ Treat like liquid solution |
$\hat{f}_i^s = X_i \Gamma_i f_i^s$ |
Phase Equilibria Applications
Vapor-liquid equilibrium (VLE)
| Description | Equations |
|---|---|
| General VLE condition | $\begin{aligned} \hat{f}_i^v &= \hat{f}_i^l \\ y_i \hat{\varphi}_i^v P &= x_i \gamma_i^l f_i^\circ \end{aligned}$ |
Raoult’s law
| Description | Equations |
|---|---|
| Raoult’s law ★ Ideal gas $\hat{\varphi}_i^v = 1$ ★ Ideal solution $\gamma_i^l = 1$ ★ Lewis/Randall ref state $f_i^\circ = f_i = P_i^{\text{sat}}$ |
$y_i P = x_i P_i^{\text{sat}}$ |
| $K$-value | $K_i = \dfrac{P_i^{\text{sat}}}{P}$ |
| Partial pressure relation of binary system | $\begin{aligned}P &= y_a P + y_b P \\ &= x_a P_a^{\text{sat}} + (1-x_a)P_b^{\text{sat}}\end{aligned}$ |
| Vapor phase composition of binary system | $y_a = \dfrac{x_a P_a^{\text{sat}}}{x_a P_a^{\text{sat}} + (1-x_a)P_b^{\text{sat}}}$ |
| Partial pressure relation of multicomponent system | $\begin{aligned}P &= \textstyle\sum y_i P \\ &= \textstyle\sum x_i P_i^{\text{sat}}\end{aligned}$ |
| Vapor phase composition of multicomponent system | $y_i = \dfrac{x_i P_i^{\text{sat}}}{\sum x_i P_i^{\text{sat}}}$ |
Nonideal liquid solution
| Description | Equations |
|---|---|
| Nonideal liquid solution ★ Ideal gas $\hat{\varphi}_i^v = 1$ ★ Lewis/Randall ref state $f_i^\circ = f_i = P_i^{\text{sat}}$ |
$y_i P = x_i \gamma_i P_i^{\text{sat}}$ |
| Partial pressure relation of binary system | $\begin{aligned}P &= y_a P + y_b P \\ &= x_a \gamma_a P_a^{\text{sat}} + (1-x_a) \gamma_b P_b^{\text{sat}}\end{aligned}$ |
| Vapor phase composition of binary system | $y_a = \dfrac{x_a \gamma_a P_a^{\text{sat}}}{x_a \gamma_a P_a^{\text{sat}} + (1-x_a) \gamma_b P_b^{\text{sat}}}$ |
| Partial pressure relation of multicomponent system | $\begin{aligned}P &= \textstyle\sum y_i P \\ &= \textstyle\sum x_i \gamma_i P_i^{\text{sat}}\end{aligned}$ |
| Vapor phase composition of multicomponent system | $y_i = \dfrac{x_i \gamma_i P_i^{\text{sat}}}{\sum x_i \gamma_i P_i^{\text{sat}}}$ |
Azeotrope
| Description | Equations |
|---|---|
| Azeotrope | $x_i = y_i$ |
| Azeotrope equilibrium consition | $P = \gamma_i P_i^{\text{sat}}$ |
| Activity coefficient from azeotrope | $\gamma_i = \dfrac{P}{P_i^{\text{sat}}}$ |
| Activity coefficient ratio from azeotrope | $\dfrac{\gamma_a}{\gamma_b} = \dfrac{P_b^{\text{sat}}}{P_a^{\text{sat}}}$ |
Objective function for modeling $\gamma_i$
| Description | Equations |
|---|---|
| Least square objective function based on pressure | $f_P = \sum (P - P_{\text{calc}})_i^2$ |
| Least square objective function based on excess Gibbs energy | $f_{g^E} = \sum (g^E - g^E_{\text{calc}})_i^2$ |
| Least square objective function based on activity coefficient for binary system | $f_\gamma = \sum \left[ \left(\dfrac{\gamma_a - \gamma_a^{\text{calc}}}{\gamma_a}\right)^2 - \left(\dfrac{\gamma_b - \gamma_b^{\text{calc}}}{\gamma_b}\right)^2 \right]_i$ |
Solubility of gases in liquids
| Ideal gas | Nonideal gas | ||
|---|---|---|---|
| Ideal liquid | Solute a | $y_a P = x_a \mathcal{H}_a$ | $y_a \varphi_a P = x_a \mathcal{H}_a \exp\left[\displaystyle\int_{P_0}^P \dfrac{\overline{V}_a^\infty}{RT} dP\right]$ |
| Solvent b | $y_b P = x_b P_b^{\text{sat}}$ | $y_b \hat{\varphi}_b P = x_b \varphi_b^{\text{sat}} P_b^{\text{sat}} \exp\left[\displaystyle\int_{P_b^{\text{sat}}}^P \dfrac{v_b^l}{RT} dP\right]$ | |
| Nonideal liquid | Solute a | $y_a P = x_a \gamma_a^\mathrm{H} \mathcal{H}_a$ | $y_a \varphi_a P = x_a \gamma_a^{\mathrm{H}} \mathcal{H}_a \exp\left[\displaystyle\int_{P_0}^P \dfrac{\overline{V}_a^\infty}{RT} dP\right]$ |
| Solvent b | $\begin{aligned} y_b P = x_b \gamma_b P_b^{\text{sat}} \end{aligned}$ | $\begin{aligned} y_b \hat{\varphi}_b P = x_b \gamma_b \varphi_b^{\text{sat}} P_b^{\text{sat}} \exp\left[\displaystyle\int_{P_b^{\text{sat}}}^P \dfrac{v_b^l}{RT} dP\right] \end{aligned}$ |
| Description | Equations |
|---|---|
| Mixing rule for Henry’s constant | $\ln\mathcal{H}_a = \sum\limits_j x_i \ln\mathcal{H}_{a, j}$ |
Liquid-liquid equilibrium (LLE)
| Description | Equations |
|---|---|
| General LLE condition | $\begin{aligned}\hat{f}_i^\alpha &= \hat{f}_i^\beta \\ x_i^\alpha \gamma_i^\alpha &= x_i^\beta \gamma_i^\beta \end{aligned}$ |
| Compositions $x_a^\alpha, x_b^\alpha, x_a^\beta, x_b^\beta$ ★ Two-suffix Margules equation |
$\begin{aligned} x_a^{\alpha}\exp \left[\dfrac{A}{RT}\left(x_b^{\alpha}\right)^2\right] &= x_a^{\beta}\exp \left[\dfrac{A}{RT}\left(x_b^{\beta}\right)^2\right] \\ x_b^{\alpha}\exp \left[\dfrac{A}{RT}\left(x_a^{\alpha}\right)^2\right] &= x_b^{\beta}\exp \left[\dfrac{A}{RT}\left(x_a^{\beta}\right)^2\right] \\ x_a^\alpha + x_b^\alpha &= 1 \\ x_a^\beta + x_b^\beta &= 1 \end{aligned}$ |
| Genral criteria for instability (separation) | $\left(\dfrac{\partial g^2}{\partial x_a^2}\right)_{T,P} < 0$ |
| Criteria for instability (separation) ★ Two-suffix Margules equation |
$\dfrac{RT}{x_ax_b} < 2A$ |
| Upper consolute temperature ★ Two-suffix Margules equation |
$T_u = \dfrac{A}{2R}$ |
Vapor-liquid-liquid equilibrium (VLLE)
| Description | Equations |
|---|---|
| General VLLE condition | $\hat{f}_i^v = \hat{f}_i^\alpha = \hat{f}_i^\beta$ |
| Composition and state variables $x_a^\alpha, x_b^\alpha, x_a^\beta, x_b^\beta, y_a, y_b, T, P$ ★ Two-suffix Margules equation |
$\begin{aligned} y_aP = x_a^{\alpha }\exp \left[\frac{A}{RT}\left(x_b^{\alpha }\right)^2\right]P_a^{\text{sat}} &= x_a^{\beta }\exp \left[\frac{A}{RT}\left(x_b^{\beta }\right)^2\right]P_a^{\text{sat}} \\ y_b P = x_b^{\alpha }\exp \left[\frac{A}{RT}\left(x_a^{\alpha }\right)^2\right]P_b^{\text{sat}} &= x_b^{\beta }\exp \left[\frac{A}{RT}\left(x_a^{\beta }\right)^2\right]P_b^{\text{sat}} \\ y_a + y_b &= 1 \\ x_a^\alpha + x_b^\alpha &= 1 \\ x_a^\beta + x_b^\beta &= 1 \end{aligned}$ |
Solid-liquid equilibrium (SLE), Solid-solid equilibrium (SSE)
| Description | Equations |
|---|---|
| General SLE condition | $\begin{aligned}\hat{f}_i^s &= \hat{f}_i^l \\ X_i \Gamma_i f_i^s &= x_i \gamma_i f_i^l \\ f_s &= x_i \gamma_i f_i^l \end{aligned}$ |
| Composition of SLE ★ Pure solid |
$\displaystyle\ln \left[x_i\gamma_i\right]=\frac{\Delta h_{\text{fus},T_m}}{R}\left[\frac{1}{T}-\frac{1}{T_m}\right]-\frac{1}{R}\int_{T_m}^T\frac{\Delta c_P^{sl}}{T}dT+\frac{1}{RT}\int _{T_m}^T\Delta c_P^{sl}dT$ |
| Composition of SLE ★ Pure solid. ★ Constant $\Delta c_P^{sl}$ |
$\displaystyle\ln \left[x_i\gamma_i\right]=\frac{\Delta h_{\text{fus},T_m}}{R}\left[\frac{1}{T}-\frac{1}{T_m}\right]-\frac{\Delta c_P^{sl}}{R}\left[1-\frac{T_m}{T}-\ln \left(\frac{T}{T_m}\right)\right]$ |
| Composition of SLE ★ Solid solution |
$\begin{aligned}\displaystyle\ln \left[\dfrac{x_i\gamma_i}{X_i \Gamma_i}\right]=\frac{\Delta h_{\text{fus},T_m}}{R}\left[\frac{1}{T}-\frac{1}{T_m}\right]-\frac{1}{R}\int_{T_m}^T\frac{\Delta c_P^{sl}}{T}dT+\frac{1}{RT}\int _{T_m}^T\Delta c_P^{sl}dT\end{aligned}$ |
| Composition of SLE ★ Solid solution. ★ Constant $\Delta c_P^{sl}$ |
$\displaystyle\ln \left[\dfrac{x_i\gamma_i}{X_i \Gamma_i}\right]=\frac{\Delta h_{\text{fus},T_m}}{R}\left[\frac{1}{T}-\frac{1}{T_m}\right]-\frac{\Delta c_P^{sl}}{R}\left[1-\frac{T_m}{T}-\ln \left(\frac{T}{T_m}\right)\right]$ |
Colligative properties
| Description | Equations |
|---|---|
| Boiling point elevation ★ Solvent $a$, solute $b$ |
$T-T_{\text{boil}}=\dfrac{RT_{\text{boil}}^2}{\Delta h_{\text{vap}}} \gamma_a x_b$ |
| Activity coefficient from boiling point elevation data | $\gamma_b=\dfrac{\left(T-T_{\text{boil}}\right)\Delta h_{\text{vap}}}{RT_{\text{boil}}^2x_b}$ |
| Freezing point depression ★ Solvent $a$, solute $b$ |
$T-T_m=\dfrac{RT_m^2}{\Delta h_{\text{fus}}}\gamma_a x_b$ |
| Activity coefficient from freezing point depression data | $\gamma_b=\dfrac{\left(T-T_m\right)\Delta h_{\text{fus}}}{RT_m^2x_b}$ |
| Osmotic pressure | $\Pi =-\dfrac{RT}{v_a}\ln \left(x_a\gamma _a\right)$ |
| Osmotic pressure ★ Ideal solution, dilute $b$ |
$\Pi =-\dfrac{RT}{v_a}x_b$ |
| Molar mass from osmotic pressure data | $\mathcal{M}_b = \dfrac{RTC_b}{\Pi}$ |
Chemical Equilibria
Single reaction equilibria
| Description | Equations |
|---|---|
| Chemical reaction expressed in stoichiometric coefficients | $\sum \nu_i A_i$ |
| Extent of reaction | $d\xi = \dfrac{dn_i}{\nu_i}$ |
| Moles of species | $n_i = n_i^\circ + \nu_i \xi$ |
| Chemical equilibrium condition | $\dfrac{dG}{d\xi} = 0 = \sum \mu_i \nu_i$ |
| Gibbs energy of reaction | $\Delta g_{\text{rxn}}^\circ = \sum \nu_i g_i^\circ$ |
| Equilibrium constant | $K = \prod \left(\dfrac{\hat{f}_i}{f_i^\circ}\right)^{\nu_i}$ |
| Equilibrium constant and Gibbs energy of reaction | $\ln K = -\dfrac{\Delta g_{\text{rxn}}^\circ}{RT}$ |
$K$ dependence on $T$
| Description | Equations |
|---|---|
| Gibbs energy of formation method | $\Delta g_{\text{rxn}}^\circ = \sum \nu_i \Delta g_{f, i}^\circ$ |
| $T$ dependence of $K$ | $\dfrac{d}{dT} \ln K = \dfrac{\Delta h_{\text{rxn}}^\circ}{RT^2}$ |
| $T$ dependence of $K$ ★ Constant $\Delta h_{\text{rxn}}^\circ$ |
$\ln \left(\dfrac{K_1}{K_2}\right)=-\dfrac{\Delta h_{\text{rxn}}^{\circ }}{R}\left(\dfrac{1}{T_2}-\dfrac{1}{T_1}\right)$ |
| $T$ dependence of $K$ ★ $\Delta h_{\text{rxn}}^\circ(T)$ |
$\ln \left(\dfrac{K_1}{K_2}\right)=-\dfrac{\Delta h_{\text{rxn}}^{\circ }}{R}\left(\dfrac{1}{T_2}-\dfrac{1}{T_1}\right) + \displaystyle\int_{T_1}^{T_2}\dfrac{\int_{T_1}^T\sum\nu_i c_{P,i}dT}{RT^2}dT$ |
$K$ dependence on concentration
Gas phase reaction
| Description | Equations |
|---|---|
| General expression | $K = \prod \left(\dfrac{y_i \hat{\varphi}_i P}{f_i^\circ}\right)^{\nu_i}$ |
| Lewis fugacity rule | $K = P^\nu \prod \left(y_i \varphi_i\right)^{\nu_i}$ |
| Ideal gas | $K = P^\nu \prod \left(y_i\right)^{\nu_i}$ |
Liquid phase reaction
| Description | Equations |
|---|---|
| General expression | $K = \prod \left(\dfrac{x_i \gamma_i f_i}{f_i^\circ}\right)^{\nu_i}$ |
| Low pressure, neglegible pressure dependence | $K = P^\nu \prod \left(x_i \gamma_i\right)^{\nu_i}$ |
| Ideal solution | $K = P^\nu \prod \left(x_i\right)^{\nu_i}$ |
Solid phase reaction
| Description | Equations |
|---|---|
| General expression | $K = \prod \left(\dfrac{X_i \Gamma_i f_i}{f_i^\circ}\right)^{\nu_i}$ |
| Low pressure, neglegible pressure dependence | $K = P^\nu \prod \left(X_i \Gamma_i\right)^{\nu_i}$ |
| Ideal solid solution | $K = P^\nu \prod \left(X_i\right)^{\nu_i}$ |
Multi-reaction equilibria
| Description | Equations |
|---|---|
| Chemical reactions expressed in stoichiometric coefficients | $\sum\limits_{k=1}^R\sum\limits_{i=1}^m\nu _{k,i}A_i$ |
| Moles of species | $n_i = n_i^\circ + \sum_{k=1}^R \nu_{k, i} \xi$ |
Electrochemical equilibria
| Description | Equations |
|---|---|
| Gibbs energy and non-Pv work | $\delta W^* \ge (dG)_{T, P}$ |
| Gibbs energy of reaction and reversible work | $W = \Delta G = z\xi FE$ |
| Nerst equation | $E=E_{\text{rxn}}^{\circ }-\dfrac{RT}{zF}\ln \left[\prod\limits_{\text{vap}}(P_i)^{\nu_i} \prod\limits_{\text{liq}}(b_i \gamma_i)^{\nu_i}\right]$ |
| Standard Gibbs energy of reaction | $\Delta g_{\text{rxn}}^{\circ }=-zFE_{\text{rxn}}^{\circ}$ |
| Standard potential of reaction | $E_{\text{rxn}}^{\circ}=-\dfrac{\Delta g_{\text{rxn}}^{\circ }}{zF}$ |
| Standard potential of reaction | $E_{\text{rxn}}^{\circ} = E_{\text{red}}^{\circ}(\text{cathode}) - E_{\text{red}}^{\circ}(\text{anode})$ |
| Average activity coefficient | $\ce{X_a Y_b <=> aX^{(z_+) +} + bY^{(z_-) -}} \newline \gamma_\pm = (\gamma_+^a \gamma_-^b)^{1/(a+b)}$ |
| Average activity coefficient | $\ce{XY <=> X+ + Y-} \newline \gamma_\pm = \sqrt{\gamma_+ \gamma_-}$ |
| Debye-Huckel model | $\ln \gamma_\pm = -A \vert z_+ z_- \vert \sqrt{I}$ |
| Ionic strength | $I = \frac{1}{2}\sum z_i^2 b_i$ |